Tau-PET Imaging Brings Braak Staging to Life
Quick Links
The hierarchical progression of tau tangles in Alzheimer’s disease—from the entorhinal cortex to the neighboring hippocampus and out into the great neocortical beyond—is emblazoned in the minds of AD researchers, in part due to Heiko and Eva Braak’s neuropathological staging scheme. The advent of ever-more-sensitive tau-PET tracers has made it possible to recapitulate this staging in living people. Now, researchers led by Pedro Rosa-Neto of McGill University in Montreal have correlated in vivo tau staging with all manner of imaging, fluid, and cognitive biomarkers. On April 25 in Nature Aging, they published more support for the idea that amyloid accumulation is a prerequisite for tangle progression beyond Braak stage III, when cognitive symptoms arise. They found that CSF biomarkers of phospho-tau start to rise as early as Braak stage II, just as tangles started to creep out of the entorhinal cortex. Their study uncovers a more nuanced, tangle-based view of biomarker trajectories in AD.
- Progression of tau tangles, as per PET, broadly followed Braak neuropathological staging.
- Amyloid plaque accumulation was necessary for Braak III or later.
- CSF p-tau biomarkers emerged in Braak stage II, with p-tau231 changing first.
“This is a very strong dataset and provides a nice framework to assess the overall progression of amyloid-related changes, soluble tau changes, fibrillar tau changes and their relationships to clinical and cognitive status,” wrote David Holtzman of Washington University in St. Louis. “It should be useful in design of clinical trials, assessing treatments, and in relating previous pathological studies to the in vivo situation in living people.” Cliff Jack, Mayo Clinic, Rochester, Minnesota, called the study “extremely well done.”
Holtzman noted that while similar studies have been done before, first author Joseph Therriault and colleagues used the PET tracer MK6240, which allows for very accurate determination of Braak staging (Mar 2016 news).
By the time memory problems arise in a person with AD, amyloid and tau pathology have been doing their dirty work for decades. This long preclinical phase of AD prompted the creation of diagnostic criteria that are based on the pathobiology of the disease (Apr 2018 news). Researchers commonly use fluid and imaging biomarkers to infer the pathological goings-on within the brain during life, while the distribution of amyloid plaques and neurofibrillary tau tangles, i.e., CERAD score and Braak staging, respectively, in brain samples serve as neuropathological gold standards postmortem (Mirra et al., 1991; Braak and Braak, 1991; Braak and Braak, 1995).
Might it be possible to stage tangle progression while people are alive? Previously, Rosa-Neto’s group had reported that [18F]MK6240, which binds tangles with subnanomolar affinity, detects their accumulation in people who were still in the presymptomatic phase of AD (Pascoal et al., 2021). In the current study, Therriault devised a PET-based version of Braak staging for 324 participants in the TRIAD cohort, tying these stages to AD biomarkers. This cohort included 179 cognitively unimpaired people, 80 with mild cognitive impairment, and 65 with AD.
Based on tracer distribution, Therriault and colleagues assigned each participant a PET-based Braak stage ranging from 0 to VI, in keeping with the neuropathological scheme. Therriault said he was surprised at how well the PET-based measures in the living cohort aligned with classical Braak staging.
People in stage 0 had no detectable tangle accumulation. In stage I, tangles were limited to the transentorhinal cortex, and in stage II they had spread into the neighboring hippocampus. In stages III and IV, tangle burden increased in these earlier stage regions, and also appeared in the temporal neocortex. Tangles cropped up in the association cortices in stage V, and extended into the primary sensory areas by stage VI.

PET Brings Braak Staging to Life. The distribution of tau tracer uptake increases hierarchically according to Braak stage. [Courtesy of Therriault et al., Nature Aging, 2022.]
By and large, tau pathology proceeded in a hierarchical fashion, meaning that tangles in later Braak stage regions only appeared in people who had tau tangles in earlier Braak stage regions, as well.
People with atypical presentations of AD deviated from this hierarchy. Some of them developed tau pathology in stage III regions before extensive tangles had built up in their stage II areas. This skewed pattern of progression dovetails with findings from a recent study by scientists in Elizabeth Mormino’s lab at Stanford University, California, who identified a subset of people with preclinical AD who had more tau in cortical regions than they did in the stage II Braak regions of the medial temporal lobe (Apr 2022 news on Young et al., 2022).
The Montreal researchers also charted the longitudinal progression of Braak staging in a subset of 163 participants who underwent follow-up scans. They found that at one- and two-year follow-up, participants either remained in the same Braak stage, or had progressed to the next. Only two people progressed through two Braak stages over two years.
How did amyloid accumulation correlate with PET-based Braak staging? The researchers found that about half of people in stage 0, I, or II were amyloid-positive as per PET. By Braak stage III, two-thirds of participants were positive for amyloid, and many of those who were negative were near the threshold for brain-wide positivity, i.e., they had some regional amyloid deposition. All participants in stage IV and beyond were amyloid-positive, and the researchers found that their amyloid burden increased up to Braak stage IV before leveling off. They came to similar conclusions when using the CSF Aβ42/40 ratio as a gauge of amyloid accumulation instead of PET. In all, these findings support the concept that amyloid accumulation is required for tau tangles to move beyond the medial temporal lobe (mTL).
Therriault also tracked CSF concentrations of four phospho-tau epitopes. Differences first emerged in Braak stage II, with small, yet detectable rises in CSF p-tau217, p-tau231, p-tau235, and p-tau181 compared to Braak stage 0. These markers ramped up significantly in Braak stage III, when tangles first appear outside of the MTL. By Braak stage IV, all four forms of p-tau started to plateau.
Interestingly, p-tau231, followed closely by p-tau217, increased the most in earlier Braak stages, while p-tau181 ramped up robustly in later Braak stages. When measured in the plasma, p-tau231 was significantly elevated by Braak II, whereas p-tau181 started to trend upward in Braak II but was not significantly up until Braak IV. Researchers in Oskar Hansson’s group at the University of Lund, Sweden, had reported similar correlations in the BioFinder cohort (Aug 2019 conference news). The early rise in p-tau231 meshes with recent findings from the ALFA cohort, which also found that this particular p-tau form rises before all others, in some cases even before brain-wide amyloid positivity, as had been reported previously (Feb 2021 news).
What about cognition? Memory problems surfaced by Braak stage II, while executive function, language, and visuospatial domains remained normal. Differences in composite measures, such as the Montreal Cognitive Assessment, did not emerge until Braak IV. Scores on the clinical dementia rating scale started to worsen in Braak III-IV, consistent with mild cognitive impairment. Most people in stage V had mild dementia, while those in stage VI had mild to moderate disease.
Therriault noted that while, on average, cognition declined with increasing Braak stage, this relationship varied from person to person. This may be because health and lifestyle factors influence cognitive decline, not just AD pathogenesis. Therriault believes the PET-based Braak staging will allow researchers to better distinguish between these risk factors, and also to investigate those that boost cognitive resilience in the face of a given pathological burden.
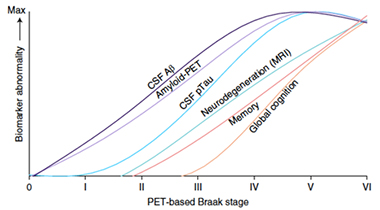
Braak During Life. Trajectories in imaging and fluid biomarkers, as well as cognition, plotted against PET-based Braak stage. [Courtesy of Therriault et al., Nature Aging, 2022.]
Pulling their data together, the researchers adapted the well-known AD staging curves described by Jack and colleagues at Mayo Clinic, Rochester (Feb 2013 conference news). Instead of plotting against time on the x-axis, as Jack had done, Therriault and colleagues plotted against PET-based Braak stage (see image below). Whether anchored in time or Braak stage, the ordering of biomarker curves looked remarkably similar.
The scientists hope that using tau PET as an unbiased anchor of pathological disease stage will allow them to validate new biomarkers, and to more thoroughly understand how existing biomarkers relate to the anatomical progression of tangles.—Jessica Shugart
References
News Citations
- Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
- New Definition of Alzheimer’s Hinges on Biology, Not Symptoms
- From Near and Far, Aβ Beckons Tau to Tangle in the Cortex
- Move Over CSF, P-Tau in Blood Also Tells Us There’s Plaque in the Brain
- Earliest of Them All: Blood P-Tau231 Assay Flags Pre-Amyloid Alzheimer’s
- HAI—Sharper Curves: Revamping a Biomarker Staging Model
Paper Citations
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479-86. PubMed.
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995 May-Jun;16(3):271-8; discussion 278-84. PubMed.
- Pascoal TA, Benedet AL, Tudorascu DL, Therriault J, Mathotaarachchi S, Savard M, Lussier FZ, Tissot C, Chamoun M, Kang MS, Stevenson J, Massarweh G, Guiot MC, Soucy JP, Gauthier S, Rosa-Neto P. Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages. Brain. 2021 Dec 16;144(11):3517-3528. PubMed.
- Young CB, Winer JR, Younes K, Cody KA, Betthauser TJ, Johnson SC, Schultz A, Sperling RA, Greicius MD, Cobos I, Poston KL, Mormino EC, Alzheimer’s Disease Neuroimaging Initiative and the Harvard Aging Brain Study. Divergent Cortical Tau Positron Emission Tomography Patterns Among Patients With Preclinical Alzheimer Disease. JAMA Neurol. 2022 Jun 1;79(6):592-603. PubMed.
Further Reading
Papers
- Biel D, Brendel M, Rubinski A, Buerger K, Janowitz D, Dichgans M, Franzmeier N, Alzheimer’s Disease Neuroimaging Initiative (ADNI). Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther. 2021 Aug 12;13(1):137. PubMed.
Primary Papers
- Therriault J, Pascoal TA, Lussier FZ, Tissot C, Chamoun M, Bezgin G, Servaes S, Benedet AL, Ashton NJ, Karikari TK, Lantero-Rodriguez J, Kunach P, Wang YT, Fernandez-Arias J, Massarweh G, Vitali P, Soucy JP, Saha-Chaudhuri P, Blennow K, Zetterberg H, Gauthier S, Rosa-Neto P. Biomarker modeling of Alzheimer's disease using PET-based Braak staging. Nat Aging. 2022 Jun;2(6):526-535. Epub 2022 Apr 25 PubMed. Nature Aging
Annotate
To make an annotation you must Login or Register.

Comments
Washington University
Therriault and colleagues assessed biomarkers in 324 individuals who were cognitively normal or had mild cognitive impairment at baseline and longitudinally. They were assessed clinically, cognitively, and for several biomarkers including with amyloid imaging, tau imaging, CSF, and plasma. Using the tau imaging agent MK6240, which allows for high resolution, accurate imaging of tau fibril pathology, they classified individuals into different Braak stages (I-IV).
Based on staging in this way, early Braak stages, e.g., stage 2, was associated with normal cognitive status or isolated memory impairment, usually CDR 0; stages III and IV with either normal cognition or mild cognitive impairment/very mild dementia (CDR 0.5); stages V and VI with very mild to higher degrees of dementia (CDR 0.5 to 2). …More
While amyloid deposition may or may not have been present in individuals up to stage III, it was always present in those with Braak stage IV or higher, emphasizing the idea that amyloid pathology drives progression of tau pathology. The authors concluded that PET-based Braak staging serves as a framework to model the natural history of AD and monitor AD severity in living humans.
I think this was a very well done study and agree with the authors’ major conclusion. Other studies have made similar observations but have not all done this with a tau imaging agent such as MK6240 that allows for very accurate determination of Braak staging.
The authors found a strong correlation with several CSF and plasma p-tau markers with Braak staging. However, they make the important point in the discussion that whether increases in soluble forms of p-tau are linked to the degree of amyloid deposition vs. the amount of tau pathology cannot be concluded from this study. For example, there are also strong correlations with the amount of amyloid deposition and CSF and plasma p-tau levels.
It seems likely from other data that soluble species of p-tau are mostly related to the amount of amyloid deposition and not the amount of tau tangles/insoluble tau. In that vein, it would have been interesting in this dataset to look at the relationship between a quantitative measure of MK6240 binding in individuals with Braak stages 0-III who were amyloid-positive or amyloid-negative. Then, in those who were amyloid-positive or amyloid-negative, is there is any relationship between the amount of p-tau species and MK6240 binding? If p-tau relates to the amount of tau pathology, one would expect the same correlation in both groups. If the correlation was much less in the amyloid-negative group, this would suggest that the relationship is driven by amyloid deposition and not tau pathology.
Overall, however, this is a very strong dataset and provides a nice framework to assess the overall progression of amyloid related changes, soluble tau changes, fibrillar tau changes and their relationships to clinical and cognitive status that should be useful in design of clinical trials, assessing treatments, and in relating previous pathological studies to the in vivo situation in living people.
Stanford University School of Medicine
Stanford University School of Medicine
This very nice study by Therriault and colleagues focuses on biomarker and cognitive changes associated with tau PET-defined Braak staging. Using a second-generation tau PET tracer with less off-target binding in choroid plexus, the authors show that increasing PET-based Braak staging is associated with increasing amyloid, increasing CSF and plasma p-tau, reductions in hippocampal volume, and cognitive decline, providing novel in vivo evidence of Braak staging with MK6240.
While the present study highlights the potential for tau PET to be used in tracking AD progression as defined by staging procedures originating from postmortem work, tau PET also offers an excellent opportunity to provide insight into the spatial patterns of tau throughout the course of disease. Therriault and colleagues highlight the exciting potential to capture focal, early tau within the EC, which has also been demonstrated by Sanchez and colleagues using flortaucipir (Sanchez et al., 2021). …More
Expanding beyond the medial temporal lobe, the authors include 11 brain regions across frontal, temporal, parietal, and occipital lobes in their definition of Braak V and four brain regions (i.e., paracentral, postcentral, precentral, pericalcarine) in their definition of Braak VI. These definitions mirror postmortem assessments, which typically sample hippocampus, entorhinal cortex (EC), middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobule, and occipital cortex (Hyman et al., 2012) to determine Braak V (defined as the presence of neurofibrillary tangles in high order association areas in frontal, parietal, occipital, and peristriate regions) and Braak VI (involvement of motor and sensory areas) staging (Braak and Braak, 1995).
The broad approach employed by Therriault and colleagues is also consistent with other recent tau PET work that has quantified tau PET SUVRs across a large cortical meta-region of interest, and with most qualitative reading scales that incorporate a cortical positive/negative dimension, with some additionally classifying the medial temporal lobe (MTL) as positive/negative (Koran et al., 2020; Sonni et al., 2020). Taken together, these studies highlight the ability to capture a progression that is consistent with Braak staging in vivo (focal EC tau, greater MTL tau, then eventually some cortical involvement), and confirm that progression along this staging framework is associated with worse clinical function.
Although tau PET imaging is limited by poor resolution and specificity, a key advantage of PET over postmortem analysis is the ability to survey the entire brain. In postmortem examination only a small set of regions are sampled, and this sampling is oftentimes only in one hemisphere (Hyman et al., 2012). Given this key difference, it is interesting that many tau PET studies have elected to summarize their data according to sparse sampling protocols used by postmortem research. Although this approach has the advantage of being hypothesis-driven and is clearly useful in distilling the data into relatively few labels (such as MTL tau positive/negative and cortical tau positive/negative as done in qualitative reads of tau PET), we are potentially overlooking spatial information that may be clinically important and may inform disease mechanisms.
Indeed, data-driven subtyping efforts have demonstrated heterogeneity in tau spatial patterns across the AD spectrum (Franzmeier et al., 2020; Vogel et al., 2021; Young et al., 2018; Young et al., 2022). Along these lines, Therriault and colleagues demonstrated that 7-11 percent of the atypical AD participants (behavioral/dysexecutive, logopenic PPA, PCA) included in their study were Braak-stage discordant, usually because there was cortical signal in the absence of elevated MTL signal. Additionally, the tau PET images of Braak-discordant cases shown in Extended Data Figure 2 demonstrate asymmetrical (in behavioral/dysexecutive AD and logopenic PPA) and precuneus-dominant (in PCA) tau patterns that are similar to the subtypes described by previous efforts (Vogel et al., 2021; Young et al., 2022).
Other work has suggested no difference in MTL uptake in atypical AD, though cortical patterns of tau elevations are clearly different in atypical presentations compared to typical AD (La Joie et al., 2021; Ossenkoppele et al., 2016; Petersen et al., 2019). These different trajectories (MTL-first pathways as well as variability in cortical patterns of spread both with and without MTL involvement) are intriguing and warrant further investigation regarding their time-course, underlying mechanisms, and clinical relevance. Overall, the full brain coverage provided by tau PET will allow us to go beyond Braak staging and characterize the different sources of tau heterogeneity.
References:
Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995 May-Jun;16(3):271-8; discussion 278-84. PubMed.
Franzmeier N, Dewenter A, Frontzkowski L, Dichgans M, Rubinski A, Neitzel J, Smith R, Strandberg O, Ossenkoppele R, Buerger K, Duering M, Hansson O, Ewers M. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer's disease. Sci Adv. 2020 Nov;6(48) Print 2020 Nov PubMed.
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012 Jan;8(1):1-13. PubMed.
Koran ME, Shams S, Adams P, Toueg T, Azevedo C, Hall J, Corso N, Sha S, Fredericks C, Greicius M, Wagner A, Zaharchuk G, Davidzon G, Chin F, Mormino E. Visual Read Protocols for Clinicians Analyzing 18F-PI-2620 tau PET/MRI Images. . Journal of Nuclear Medicine, May 1, 2020 Journal of Nuclear Medicine
La Joie R, Visani AV, Lesman-Segev OH, Baker SL, Edwards L, Iaccarino L, Soleimani-Meigooni DN, Mellinger T, Janabi M, Miller ZA, Perry DC, Pham J, Strom A, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Association of APOE4 and Clinical Variability in Alzheimer Disease With the Pattern of Tau- and Amyloid-PET. Neurology. 2021 Feb 2;96(5):e650-e661. Epub 2020 Dec 1 PubMed.
Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016 May;139(Pt 5):1551-67. Epub 2016 Mar 8 PubMed.
Petersen C, Nolan AL, de Paula França Resende E, Miller Z, Ehrenberg AJ, Gorno-Tempini ML, Rosen HJ, Kramer JH, Spina S, Rabinovici GD, Miller BL, Seeley WW, Heinsen H, Grinberg LT. Alzheimer's disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 2019 Oct;138(4):597-612. Epub 2019 Jun 27 PubMed.
Sanchez JS, Becker JA, Jacobs HI, Hanseeuw BJ, Jiang S, Schultz AP, Properzi MJ, Katz SR, Beiser A, Satizabal CL, O'Donnell A, DeCarli C, Killiany R, El Fakhri G, Normandin MD, Gómez-Isla T, Quiroz YT, Rentz DM, Sperling RA, Seshadri S, Augustinack J, Price JC, Johnson KA. The cortical origin and initial spread of medial temporal tauopathy in Alzheimer's disease assessed with positron emission tomography. Sci Transl Med. 2021 Jan 20;13(577) PubMed.
Sonni I, Lesman Segev OH, Baker SL, Iaccarino L, Korman D, Rabinovici GD, Jagust WJ, Landau SM, La Joie R, Alzheimer's Disease Neuroimaging Initiative. Evaluation of a visual interpretation method for tau-PET with 18F-flortaucipir. Alzheimers Dement (Amst). 2020;12(1):e12133. Epub 2020 Nov 28 PubMed.
Vogel JW, Young AL, Oxtoby NP, Smith R, Ossenkoppele R, Strandberg OT, La Joie R, Aksman LM, Grothe MJ, Iturria-Medina Y, Alzheimer’s Disease Neuroimaging Initiative, Pontecorvo MJ, Devous MD, Rabinovici GD, Alexander DC, Lyoo CH, Evans AC, Hansson O. Four distinct trajectories of tau deposition identified in Alzheimer's disease. Nat Med. 2021 May;27(5):871-881. Epub 2021 Apr 29 PubMed.
Young AL, Marinescu RV, Oxtoby NP, Bocchetta M, Yong K, Firth NC, Cash DM, Thomas DL, Dick KM, Cardoso J, van Swieten J, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni GB, Laforce R Jr, Finger E, de Mendonça A, Sorbi S, Warren JD, Crutch S, Fox NC, Ourselin S, Schott JM, Rohrer JD, Alexander DC, Genetic FTD Initiative (GENFI), Alzheimer’s Disease Neuroimaging Initiative (ADNI). Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018 Oct 15;9(1):4273. PubMed.
Young CB, Winer JR, Younes K, Cody KA, Betthauser TJ, Johnson SC, Schultz A, Sperling RA, Greicius MD, Cobos I, Poston KL, Mormino EC, Alzheimer’s Disease Neuroimaging Initiative and the Harvard Aging Brain Study. Divergent Cortical Tau Positron Emission Tomography Patterns Among Patients With Preclinical Alzheimer Disease. JAMA Neurol. 2022 Jun 1;79(6):592-603. PubMed.
Make a Comment
To make a comment you must login or register.