Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
Quick Links
Tau accumulates in the brain in Alzheimer’s disease, but also during normal aging. Can PET scans tell the difference? Two recent papers say yes, and propose schemes for imaging-based staging that until now was possible only at autopsy. As reported in the March 2 Neuron, scientists led by Bill Jagust at the University of California, Berkeley, used the tau PET ligand AV1451 to reveal an age-related accumulation of tau in the medial temporal lobe in healthy older adults that tracks with weakening episodic memory. When amyloid enters the picture, however, things ramp up. Tau binding extends beyond the medial lobe and higher up into the cerebral cortex in AD, while cognition declines more broadly. Imaging enabled the scientists to stage disease severity in vivo, closely matching the stages predicted by neuropathology. A second group of scientists also reported that they have recapitulated disease staging with tau imaging in vivo. Led by Adam Schwarz at Eli Lilly and Company, Indianapolis, and Mark Mintun at Avid Pharmaceuticals, Philadelphia, that paper appeared in the March 2 issue of Brain.
“Imaging studies will help clarify the contribution of amyloid and tau pathology to cognition, normal aging, and Alzheimer’s dementia,” said Karen Duff, Columbia University, New York, who was not involved in the work. “I am happy to see how useful tau PET imaging is going to be in staging and monitoring of disease, which will contribute greatly to diagnosis and drug development.”
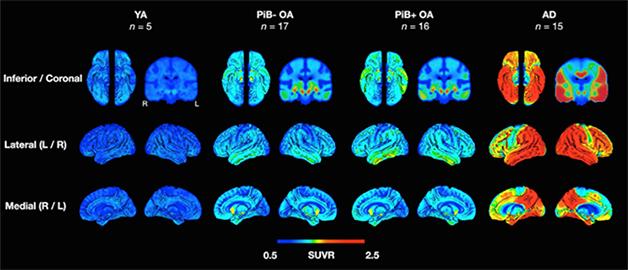
Tau Progression. In young adults (YA), older adults (OA) with and without amyloid, and people with AD, uptake patterns of a tau tracer match Braak staging. [Courtesy of Neuron, Schöll et al.]
Presented at two recent conferences (Aug 2015 conference news; Feb 2015 conference news), Jagust’s findings corroborate histopathological and imaging data from other labs. Previous postmortem data suggested that tau collects in the medial temporal lobe (MTL) as people age, in what some call primary age-related tauopathy, or PART (Braak et al., 2011; Crary et al., 2014). Tau seems to branch outside of those areas only when amyloid enters the picture (Price and Morris, 1999). PET now bears that out, and it does so with the great advantage of allowing scientists to view the whole brain at once, in vivo, said Aaron Schultz, Massachusetts General Hospital, Boston. Schultz was among scientists in the lab of Keith Johnson, who recently reported an age-related uptake of AV1451 in the inferior temporal lobe (Johnson et al., 2016). Johnson’s group found that AV1451 uptake in the neocortex coincided with higher Aβ burden, and that this predicted more severe clinical impairment.
In Jagust’s lab, co-first authors Michael Schöll and Samuel Lockhart enrolled five healthy young adults between the ages of 20 and 26, another 33 cognitively healthy older adults aged 64 to 90, and 15 patients aged 53 to 77 with a diagnosis of probable AD (McKhann et al., 2011). All participants, except the young healthy adults, underwent PET with Pittsburgh compound B (PiB-PET) to detect brain amyloid, and AV1451 to measure tau. They also took tests of episodic memory, working memory, and executive function/processing speed. Many of the healthy older adults had sat these neuropsychological tests up to six years prior to their scans.
PET indicated that the young healthy adults had accumulated very little tau (see image above). Older people who were negative for amyloid had tau aggregates in the MTL that correlated with subtle deficits in episodic memory but not other forms. In older adults who did have brain amyloid, tau appeared in the inferior plus the lateral temporal lobe, as well as in some lateral and medial parietal regions. AD patients had tau pathology in most neocortical regions, particularly throughout temporal, parietal, and frontal lobes. Tau pathology outside the MTL correlated with a drop in global cognition, but only if amyloid was present. “It’s a pretty clear story: As you get older, there’s more tau in your medial temporal lobe, but as you get more amyloid, there’s more tau in your lateral temporal lobe and your neocortex,” Jagust told Alzforum.
Jagust said it will be difficult for imaging to determine whether amyloid causes tau to spread, or whether the spread of tau allows amyloid to deposit. Likewise, imaging will probably be unable to say whether PART and AD represent continuous or different processes, he said.
“Ultimately, by [therapeutically] targeting amyloid we may change what happens with tau,” said Schultz. “Being able to image that in vivo will be immensely useful, allowing better sensitivity and earlier assessment than would be possible with behavioral measures alone.” He noted that the current data is still cross-sectional. “One big hope is that new insights into disease will come from longitudinal data. A great many people are excited about that.”
Taking the results a step further, Schöll worked out a method for in vivo Braak staging (see Braak and Braak, 1991). It defines seven stages of tau progression based on histopathology: no deposition in stage 0; the transentorhinal region in stage I; entorhinal cortex in stage II; fusiform and lingual gyri in stage III; neocortical association areas by IV. By stage V/VI, tangles have expanded widely into the outer layers of the cortex.
Schöll adapted this staging scheme for in vivo tau PET by defining three large regions of interest that matched Braak stages I/II, III/IV, and V/VI. He then used regression models to define standard uptake value ratio thresholds for each region that indicated whether a person was positive for tau (see image below) in that region. Those values classified all of the young adults and two older adults as stage 0, 25 older adults as I/II, two AD patients and six older adults as III/IV, and 13 AD patients as stage V/VI. As expected, higher Braak stage correlated with increasing, global amyloid burden.
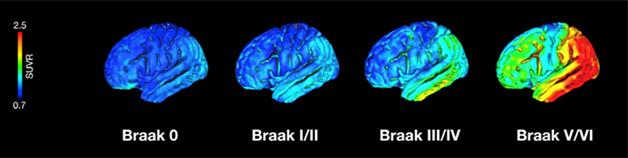
Braak Stages Come to Life. Researchers can now classify people by Braak stages according to in vivo measurements of tau accumulation. [Courtesy of Neuron, Schöll et al.]
“Tau PET is offering the possibility of staging Alzheimer’s disease in vivo,” said Schöll. Now researchers can classify living people by cognitive, amyloid, and tau profiles. This will help them predict how close a person is to progressing to AD. In turn, this will be helpful in recruiting better-characterized participants for clinical trials, and eventually for diagnosis, he said.
Schwarz, Mintun, and colleagues took a slightly different approach to staging disease in people (Schwarz et al., 2016). They used AV1451 to scan 14 young controls and 42 cognitively normal older adults who were free of amyloid (according to a florbetapir scan), as well as 87 patients with mild cognitive impairment (MCI) and 44 with a clinical diagnosis of AD, 16 of whom tested negative for amyloid. Where Schöll defined three large ROIs to tau staging, Schwarz chose six, one for each individual Braak stage. He chose cutoffs for each region that exceeded the uptake level he saw in young healthy controls.
Schwarz found that his in vivo staging largely matched that expected for each person’s clinical profile. Worse cognitive impairment, more advanced disease stage, and amyloid positivity associated with higher stages. However, fewer cognitively normal older adults were rated stage I/II than expected based on historical histopathology data. The researchers took this to mean that tau PET imaging is less sensitive than staining methods. They also found that 7 percent of their population did not show typical patterns of tau distribution—three older cognitively normal people, eight with MCI (four amyloid-positive), and one AD patient. Several retained no AV1451 in the medial and inferior temporal lobe but had strong signals in lateral temporal and/or occipital cortices, which could be related to a proposed hippocampal-sparing subtype of AD, the authors wrote (Murray et al., 2011). They suggested that the in vivo staging will eventually be refined by comparing in vivo PET imaging with postmortem neuropathology of the same study volunteers.—Gwyneth Dickey Zakaib
References
News Citations
- New Imaging Data Tells Story of Travelling Tau
- Tau Tracer T807/AV1451 Tracks Neurodegenerative Progression
Paper Citations
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-9. PubMed.
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014 Dec;128(6):755-66. Epub 2014 Oct 28 PubMed.
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999 Mar;45(3):358-68. PubMed.
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016 Jan;79(1):110-9. Epub 2015 Dec 15 PubMed.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011 May;7(3):263-9. PubMed.
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
- Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, Joshi AD, Devous MD Sr, Mintun MS. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016 May;139(Pt 5):1539-50. Epub 2016 Mar 2 PubMed.
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011 Sep;10(9):785-96. PubMed.
Further Reading
Primary Papers
- Schöll M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016 Mar 2;89(5):971-82. PubMed.
- Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, Joshi AD, Devous MD Sr, Mintun MS. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016 May;139(Pt 5):1539-50. Epub 2016 Mar 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
KULeuven
Very good to learn that protein tau is making more headlines in the AD field and that ardent baptists see the "tau-light." We just added our contribution to tau diagnostics, see below. The data is in mouse models for the time being. There can never be enough tools nor data in the battle against Alzheimer's disease and the many other tauopathies.
References:
Brendel M, Jaworska A, Probst F, Overhoff F, Korzhova V, Lindner S, Carlsen J, Bartenstein P, Harada R, Kudo Y, Haass C, Van Leuven F, Okamura N, Herms J, Rominger A. Small-Animal PET Imaging of Tau Pathology with 18F-THK5117 in 2 Transgenic Mouse Models. J Nucl Med. 2016 May;57(5):792-8. Epub 2016 Feb 11 PubMed.
MRC Laboratory of Molecular Biology
In science, it often pays to see what is in front of you, without being encumbered by preconceptions. The pivotal 1991 study by Heiko Braak and Eva Braak did just that (Braak and Braak, 1991). It showed that tau inclusions form in a stereotypical fashion, allowing classification depending on where the inclusions are found and in what number. Cross-sectional autopsy studies of argyrophilic tau pathology showed first deposits in transentorhinal cortex (stages I/II), followed by hippocampus (stages III/IV) and neocortex (stages V/VI). These findings suggested that the biological process of Alzheimer’s disease begins decades before clinical symptoms appear. They are also compatible with the view that argyrophilic tau inclusions first appear in the transentorhinal cortex, from where they spread to distant brain regions, given enough time. In recent years, much experimental evidence has been adduced in favor of the spreading of tau inclusions from an initial site of formation (Goedert, 2015). What causes the formation of tau inclusions at this site is not known.
The development of tau inclusions in transentorhinal cortex and hippocampus may be necessary (but not sufficient) for Alzheimer’s disease (Braak and Del Tredici, 2015; Duyckaerts et al., 2015). The latter is always present when abundant Aβ and tau deposits have formed in neocortex (Price and Morris, 1999). The lack of topological correlation between both types of deposits has suggested that they may form independently, but that neocortical Aβ deposits may come to drive the development of tau inclusions and may cause the spread of tau pathology. This interpretation is compatible with the amyloid cascade hypothesis, which was put forward around the time Braaks’ paper was published (Hardy and Allsop, 1991). However, it remains to be seen if stereotypical tau staging also applies to dominantly inherited cases of Alzheimer’s disease with mutations in APP; it was the study of those cases that led to the amyloid cascade hypothesis.
Until recently, the anatomical distribution of tau inclusions could not be mapped directly in living individuals. This has changed with the arrival of PET tracers specific for aggregated tau. 18F-AV-1451 binds preferentially to tau filaments from Alzheimer’s disease brain, but not to Aβ, α-synuclein, or TDP-43 deposits. It came out of compound library screens that used brain sections from Alzheimer’s disease patients (Xia et al., 2013). Tau filaments from Alzheimer’s disease brains are made of all six human brain isoforms. The same is the case of tau filaments that form as function of age; it is also true of a number of other tauopathies, including chronic traumatic encephalopathy, tangle-only dementia, Niemann-Pick disease type C, the parkinsonism-dementia complex of Guam, cases of Gerstmann-Sträussler-Scheinker disease, and some cases with MAPT mutations (such as V337M and R406W). 18F-AV-1451 may be of use in all these conditions. However, it may not be of sufficient sensitivity in progressive supranuclear palsy and corticobasal degeneration, where tau filaments only comprise isoforms with four repeats, or in Pick’s disease, where tau isoforms with three repeats predominate in the filaments (Marquié et al., 2015). This imaging tool makes it possible to stage tau pathology longitudinally and across the disease spectrum. Prior to the current studies, PET scans had already shown a significantly greater retention of 18F-AV-1451 in the brains of patients with Alzheimer’s disease compared to individuals with mild cognitive impairment and controls (Chien et al., 2013; Johnson et al., 2016).
The new studies by Schöll et al. and Schwarz et al. show that 18F-AV-1451 PET images from subjects aged 50-95 years with or without Alzheimer’s disease can be classified into patterns similar to those described by the Braaks in their neuropathological staging of tau pathology. By applying an algorithm based on the histological staging procedure, it has been possible to estimate Braak stages directly from PET scans. However, the relationship between Braak stages assigned by in vivo imaging and postmortem neuropathology remains to be defined. Both methods are likely to look at the same structures, paired helical and straight tau filaments, but they may differ in sensitivity. Our recent findings (Jackson et al., 2016) have shown that short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. If the same holds true of aging and Alzheimer’s disease, increasing Braak tau stages may reflect the spreading of inclusions.
Young adults showed only minimal brain uptake of ligand, consistent with Braak stage 0. Most older adults were at Braak stages I/II (76 percent) and a smaller proportion was at Braak stages III/IV (18 percent) or Braak stage 0 (6 percent). Patients with clinical Alzheimer’s disease were predominantly at stages V/VI (87 percent). However, a minority (13 percent) was at stages III/IV, showing an overlap with the changes seen in some older individuals without Alzheimer’s disease. No cases of Alzheimer’s disease were at stages I/II. The studies of Braak and colleagues had shown previously that individuals at stages I/II of tau pathology did not have cognitive impairment, whereas those at stages V/VI had Alzheimer’s disease clinically. Assuming that there is a continuum between stages, it follows that preventing the spreading of tau inclusions from stages I/II to stages V/VI might well prevent disease symptoms. It will be important to see if, using PET imaging with 18F-AV-1451, tau pathology progresses over time from stages I/II to stages III/IV in a given individual. It will be equally interesting to find out if stages III/IV are a necessary prerequisite for the development of stages V/VI.
References:
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain. 2015 Oct;138(Pt 10):2814-33. Epub 2015 Aug 17 PubMed.
Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Shankle WR, Elizarov A, Kolb HC. Early Clinical PET Imaging Results with the Novel PHF-Tau Radioligand [F-18]-T807. J Alzheimers Dis. 2013 Jan 1;34(2):457-68. PubMed.
Duyckaerts C, Braak H, Brion JP, Buée L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M, Uchihara T. PART is part of Alzheimer disease. Acta Neuropathol. 2015 May;129(5):749-56. Epub 2015 Jan 28 PubMed.
Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015 Aug 7;349(6248):1255555. PubMed.
Jackson SJ, Kerridge C, Cooper J, Cavallini A, Falcon B, Cella CV, Landi A, Szekeres PG, Murray TK, Ahmed Z, Goedert M, Hutton M, O'Neill MJ, Bose S. Short Fibrils Constitute the Major Species of Seed-Competent Tau in the Brains of Mice Transgenic for Human P301S Tau. J Neurosci. 2016 Jan 20;36(3):762-72. PubMed.
Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016 Jan;79(1):110-9. Epub 2015 Dec 15 PubMed.
Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Vasdev N, Dickerson BC, Gomperts SN, Growdon JH, Johnson KA, Frosch MP, Hyman BT, Gómez-Isla T. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015 Nov;78(5):787-800. Epub 2015 Sep 25 PubMed.
Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999 Mar;45(3):358-68. PubMed.
Schöll M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016 Mar 2;89(5):971-82. PubMed.
Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, Joshi AD, Devous MD Sr, Mintun MS. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016 May;139(Pt 5):1539-50. Epub 2016 Mar 2 PubMed.
Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Su H, Szardenings AK, Walsh JC, Wang E, Yu C, Zhang W, Zhao T, Kolb HC. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 2013 Feb 11; PubMed.
Make a Comment
To make a comment you must login or register.