Move Over CSF, P-Tau in Blood Also Tells Us There’s Plaque in the Brain
Quick Links
From day one at this year’s AAIC, held July 14-18 in Los Angeles, conference halls and corridors buzzed with the sound of “p-tau.” Scientists from Randall Bateman’s lab at Washington University, St. Louis, reported that among volunteers in DIAN, certain species of phospho-tau inched up in the CSF as early as 21 years before symptom onset, many years before tangles can be detected in the brain by PET imaging. Niklas Mattsson from the BioFINDER group at Lund University, Sweden, showed that the same species, p181- and p217-tau, rose in the CSF even before plaques can be detected by PET (see Part 7 of this series). Researchers entertained the prospect of getting information on both early amyloid and early tau changes from one lumbar puncture.
- In CSF, p181- and p217-tau tick up very early in AD.
- Immunoassays detect p181-tau in plasma.
- P181-tau could be a blood marker of early amyloidosis.
What about blood? Given the recent success in developing plasma assays for Aβ that reflect ongoing brain amyloid, scientists wanted to know if this p-tau-amyloid link would hold up for a person’s blood as well. And there are tools to find out. Jeffrey Dage at Lilly Research Laboratories, Indianapolis, has developed an in-house antibody test for plasma p181-tau. Scientists at the University of Gothenburg are working on one, also.
Last year, Michelle Mielke and colleagues at the Mayo Clinic, Rochester, Minnesota, sent samples from their Mayo Clinic Study of Aging and from the Alzheimer’s Disease Research Center at Mayo to Dage for testing. Voila—increases in plasma p181-tau turned up, and tracked with AD severity (Mielke et al., 2018). In Los Angeles, Mielke was delighted to learn that other groups described complementary data.
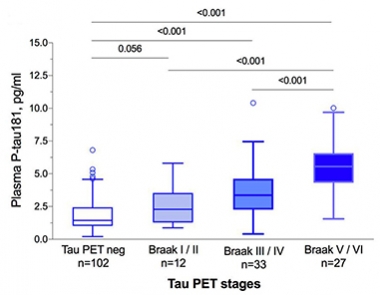
In Synch. Levels of p181-tau in the plasma track with Braak staging as determined by tau PET. [Courtesy of Oskar Hansson.]
For example, Oskar Hansson and Shorena Janelidze from the BioFINDER group at Lund University, reported a tight correlation between plasma p181-tau and p181-tau in the CSF of amyloid-positive participants in this longitudinal cohort. Moreover, plasma p181-tau tightly correlated with flortaucipir uptake in the brain and predicted Braak staging, Hansson said (see image below).
To be sure, the concentration of tau in blood seems vanishingly small, but it is detectable. Average plasma levels of 1.25 pg/mL in tau PET negative controls compared with about 2.5, 3.8, and 6 pg/mL in those with tau PET scans indicative of Braak stages I/II, III/IV, and V/VI, respectively. This suggests this plasma marker could help with both diagnosis and staging.
In keeping with this idea, plasma p181-tau poorly correlated with flortaucipir in Aβ-negative people. Levels of p181-tau in plasma from people with a non-AD dementia didn’t budge above baseline, either, suggesting this marker might be quite specific for AD and help with differential diagnosis. Plasma p181-tau differentiated AD from other neurodegenerative disorders with an AUC of 0.95, a similar accuracy to tau PET and CSF p181-tau.
Elisabeth Thijssen, University of California, San Francisco, came to much the same conclusion. She compared plasma p181-tau and neurofilament light as potential markers for AD and frontotemporal dementias. Tau marker levels were higher in 39 AD patients than in any of 45 normal controls, 40 MCI patients, or 143 FTLD cases. Among the latter group, 36 people with corticobasal syndrome, 47 with progressive supranuclear palsy, 46 with behavioral variant FTD, and 14 patients with primary progressive aphasia all posted similar p181-tau levels as did controls. Plasma p181-tau distinguished these FTLD cases from AD with about 90 percent accuracy, Thijssen reported.
Why did the MCI group not have higher plasma p181-tau if this marker rises so early in AD pathogenesis? It turns out 21 of those 40 MCI cases were amyloid-negative. The amyloid-positive cases, on the other hand, did have three times more p181-tau in their plasma. Indeed, the marker identified amyloid PET-positives among the MCI group with 95 percent accuracy.
Like Hansson, Thijssen also found that plasma p181-tau correlated tightly with flortaucipir uptake in amyloid-positive people. Among 72 AD/MCI patients, plasma p181-tau also correlated tightly with atrophy of the temporo-parietal region of the brain, including the medial temporal lobe, the posterior cingulate cortex, and the precuneus.
Hansson believes that plasma p181-tau not only distinguishes AD from most non-AD dementias, but that it can also predict future development of AD. In a survival analysis, he found that for each standard deviation increase in this blood marker in a given group, their incidence of future AD rose 2.8-fold. And as a prognostic marker, p181-tau outshone other plasma markers in a multivariate analysis, including Aβ42/40, total tau, and NfL, none of which associated with risk of AD, he said. Hansson thinks plasma p181-tau, much like p181-tau in CSF, will be shown to nudge above baseline levels even before amyloid can be detected by PET imaging, and he foresees p181-tau being used as a very early marker of AD. He predicted a combination of plasma Aβ42/40 ratio and p181-tau might be optimal for detection of AD pathology during the early disease stages.
Paul Aisen, University of Southern California, San Diego, thought that p181-tau looked extremely interesting, but stopped short of calling it a marker of amyloidosis, as some suggested. “I think the better test for amyloid is the plasma Aβ42/40 ratio, particularly in presymptomatic individuals,” he said (for news on plasma Aβ42/40, see Parts 9 and 10 of this series).
Adam Boxer, University of California, San Francisco, agreed. “I think p181- tau reflects overproduction of tau in the AD state, but it is not a marker of plaques,” he told Alzforum. Boxer noted that p181-tau ticks up in certain FTLD patients who accumulate neurofibrillary tangles containing both three- and four-repeat tau—the same type of tangle found in AD. Some rare FTLD tau mutations, such as R406W, cause 3R/4R tangles. “I think p181 may be a marker for 3R/4R tangles, said Boxer.
This would agree with Mattsson’s data, which suggest that phosphorylation at threonines 181 and 217 associates with tangle formation. He used PET and CSF data to model how soluble forms of tau mediate the relationship between amyloid and tangles. To ensure he was capturing early changes, Mattsson analyzed data from people who were cognitively normal or only slightly impaired. He calculated that the direct effect of amyloid on tangles was small; rather, p217- and p181-tau mediated 68 and 80 percent of the effect, respectively.
Given this mediation, and their very early rise in AD, are p181- and p217-tau markers of tau processing rather than of tangles? “That’s the implication here. I would be surprised. I think we need to see more data on that,” said Aisen. Hansson also cautioned against overinterpretation. “We have a sensitivity issue with PET,” he said. “We can’t be sure that the p-tau increases do not come after someone has already got a handful of tangles in their brain that PET is not sensitive enough to detect.”
Noting that fluid tests tend to be more sensitive than PET, Kaj Blennow, University of Gothenburg, drew an analogy between the current state of p-tau research and the early days of Aβ42 CSF/amyloid PET concordance studies. Those studies found people who were CSF-positive but PET-negative, and these people became PET-positive over time. “That is accepted now,” said Blennow, “and the same could be the case with plasma tau.” In other words, if plasma and CSF p181-tau tests are more sensitive than tau PET, then people who are now testing positive on p181-tau fluid tests but negative on PET may well be positive on both in a few years.
This also means that the staging diagram as currently perceived may reflect differential sensitivity of the markers as much as true biological sequence of change, Blennow said.
Many researchers at AAIC suggested that plasma p181-tau might prove to be less fickle than the plasma Aβ42/40 ratio. The latter falls by only 10 to 15 percent in AD versus controls, and fluctuations caused by environmental factors such as sleep, exercise, and cardiovascular fitness might make that differential smaller still. In contrast, the change in p-tau is an order of magnitude above controls, and continues to climb with disease progression. “Yes, the differences in the Aβ ratio test may be small, but we are still able to achieve a robust distinction between normal and abnormal,” countered Aisen. “I think p181-tau is a measure of disease stage and of neurodegeneration, and is going to be extremely useful for tracking progression,” he said. Aisen believes it is time to start incorporating plasma markers into clinical studies. “Maybe p-tau is even better than NfL,” he suggested.
For her part, Thijssen might agree, at least on the differential diagnosis. She found that unlike p181-tau, plasma NfL levels were indistinguishable between normal controls and AD patients, while NfL levels in CBS, PSP, and bvFTLD patients were at least twofold higher than controls. For AAIC news on plasma Aβ, see part 9 of this series. —Tom Fagan
References
News Citations
- Move Over Aβ, CSF P-Tau Tells Us There’s Plaque in the Brain
- Are Aβ Blood Tests Ready for Prime Time?
- Why Bother With Round Robins on Blood Tests? Q&A with Kaj Blennow
Research Models Citations
Paper Citations
- Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, Jack CR Jr, Petersen RC, Dage JL. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018 Aug;14(8):989-997. Epub 2018 Apr 5 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.