No Special Glasses Needed: Three-Dimensional Views of Tau and Aβ in the Brain
Quick Links
A picture may be worth more than a thousand words if it is three-dimensional. Two recent papers take viewers inside the whole brains of transgenic mice to reveal the complexities of abnormal tau and Aβ plaques in all dimensions. In the July 28 PLoS ONE, scientists led by Hongjun (Harry) Fu and Karen Duff, Columbia University Medical Center, New York, chart the spread of human tau in a mouse that expresses the protein predominantly in the entorhinal cortex. The researchers track pathological forms of tau through the hippocampus to connected areas in the neocortex, leaving cell death and cognitive decline in its wake. “It validates the idea that neurotoxicity is associated with the spread of tauopathy,” Duff told Alzforum. Appearing July 26 in Cell Reports, a paper from scientists led by Marc Flajolet, Rockefeller University, New York, describes a similar three-dimensional reconstruction of Aβ plaques pervading the brains of mouse models of AD and of postmortem human brain tissue. They find that plaques form larger, complex structures in people that are not found in the mice. “It’s clearly a large type of organization that we have not seen before,” said Flajolet. If these patterns are found in more patients, they may help categorize different forms of disease, he suggested.
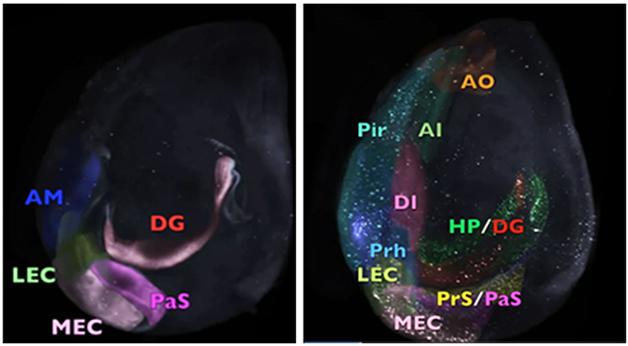
Three-Dimensional Videos: In mice that express human tau in the entorhinal cortex (EC), granules of tau appear first in the EC (eight-month-old animals, on left), then other areas of the neocortex (34-month-old animals, right). Distinct brain regions harboring human tau pathology are artificially colored, while areas without are clear. See full videos below. [Courtesy of Fu et al.]
In a process called iDISCO—short for immunolabeling-enabled three-dimensional imaging of solvent cleared organs—scientists use solvents to wipe brains clear of the lipids that make them opaque. The process leaves behind a transparent, glass-like brain, with its vasculature, cells, and even synapses all intact. A variety of imaging techniques can then illuminate what’s inside. Light sheet microscopy, for instance, shoots a flat laser beam through the tissue, capturing pictures of entire swaths of the brain at once. The technique joins other tissue clearing methods such as CLARITY (Apr 2013 news) and Scales (see Sep 2015 news) that have been used to peer deep inside brains.
In Duff’s lab, first author Fu and colleagues wanted to visualize the three-dimensional spatial and temporal spread of tauopathy over the lifespan of mice developed by Duff and others to mimic the stages of tauopathy seen in AD. He used the EC-tau mouse, in which the neuropsin gene promoter, which is active predominantly in the entorhinal cortex (EC), regulates expression of a tetracycline receptor, which then turns on the P301L version of the human tau gene once the animals are given tetracycline (Feb 2012 news). “We wanted to see more clearly how tau pathology matures and spreads as the mouse ages,” said Fu. He allowed the mice to live longer than any previous study, sacrificing animals at eight, 14, 24, and 34 months for brain clearing.
After infusing the brains with the CP27 antibody, which recognizes only human tau, and then clearing the tissue by iDISCO, Fu and colleagues used light sheet microscopy to locate tau. At eight months, the protein had begun to build up in neuronal bodies (soma) and dendrites of the medial entorhinal cortex and parasubiculum (see first video below). Duff considers somatodendritic tau a sign of ongoing tau pathology, since the protein is usually confined to axons in normal cells (see Jan 2011 news). By 14 months, tau had accumulated in the somatodendritic compartments of neurons in the dentate gyrus and CA1 of the hippocampus. At 24 months, it appeared in the presubiculum, subiculum, anterior cingulate cortex, perirhinal cortex, and piriform cortex. At the oldest time point tested, the tauopathy was abundant in the neocortical areas, the olfactory system, and the amygdala (see second video below). “This mouse model clearly shows that pathology in the amygdala is linked in some way to tauopathy coming from the hippocampal formation,” said Duff.
In contrast, control mice that did not express the tau transgene accumulated no appreciable somatodendritic tau, indicating that the widespread pathology was initiated by human tau in the EC. “This is the first time that pathology in the hippocampal formation of a mouse has been shown to spread out into the neocortex,” said Duff. “That’s very important for our understanding of the disease, because that’s exactly what happens in humans.”
To confirm what they saw in three dimensions, the authors then prepared slices of tissue for a typical two-dimensional inspection. Staining with the MCI antibody, which detects pathological conformations of human tau, revealed pathology in the same brain regions at the same ages seen in three-dimensional imaging.
Did that pathology affect the cellular environment? To find out, Fu and colleagues stained two-dimensional slices for activated microglia, reactive astrocytes, and neurons. At 14 months, activated microglia and reactive astrocytes were piling up in the medial entorhinal cortex. Those cells grew in number as mice got older, and appeared in other areas, including the amygdala, when tau pathology reached there. In 34-month-old mice, NeuN-positive neurons started to die in the vicinity of the tau pathology in the entorhinal cortex and later in the parasubiculum. This suggests cells succumb first in areas of the most overt pathology, then in areas where it spreads. This neuronal death followed soon after the mice showed signs of memory loss. Thirty–month-old mice spent as much time exploring a novel object as a familiar one, suggesting that long-term memory had started to fail. Once again, these patterns parallel Alzheimer’s disease, where cognitive deficits accompany the spread of tauopathy outside the medial temporal lobe, Duff pointed out. “We think we’ve captured the stage of progression into the cognitive decline phase of AD disease in this mouse model.”
Lary Walker, Emory University, Atlanta, said the data suggest that tauopathy accelerates in these old mice, negatively affecting behavior. “This method allows for efficient three-dimensional imaging of small brain regions compared to the relatively laborious method of sectioning, staining and reconstructing to achieve 3-D,” he wrote to Alzforum. “I expect this methodology will be used much more to assess spatial relationships among lesions, and to track the progression of neurodegenerative processes.”
Interestingly, because Fu found no tau in microglia or astrocytes in these mice, the authors think uptake of pathological tau into these cells is unlikely to underlie the spread of tauopathy in the brain as others have proposed—at least in this EC-tau mouse model (Oct 2015 news). Tsuneya Ikezu, Boston University, pointed out that immune cells and microglia are not equipped to store proteins they ingest. “The lack of accumulation of tau in microglia does not disprove their role in phagocytosis of neurons or pathogenic proteins,” he wrote to Alzforum (see full comment below).
In the second paper, first author Thomas Liebmann and colleagues used the iDISCO procedure to clear the brains of 2xTg AD mice, which express mutant APP and PSEN1. In animals sacrificed at five time points between 4.4 and 27 months of age, they labeled up to three parameters at a time—amyloid plaques with Congo red or the commercial antibody PAB #10, tau with Tab #7, cell nuclei with TO-PRO-3, microglia with an anti-Iba1 antibody, and blood vessels by autofluorescence (see image below). The researchers selected these antibodies after testing for ability to penetrate and label deep tissues.
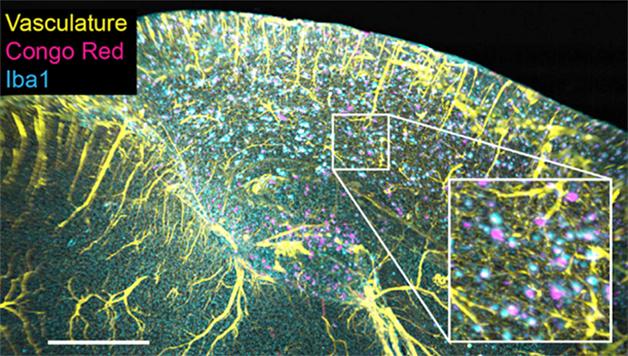
A Colorful Trio: In the brain of the 11-month-old 2xTg mouse, amyloid plaques (pink) are surrounded by microglia (blue) and appear near blood vessels (yellow). [Cell Reports, Liebmann et al.]
The authors saw a steady progression of tau tangles starting in the cortex at four months, increasing in the cerebellum and hippocampus by 6.5, and to the thalamus and striatum by 10 and 17 months respectively. After confirming their findings with two-dimensional staining, they found they could detect plaques earlier in three dimensions. They claim sparse plaques are less likely to appear in any given two-dimensional slice. Each plaque was ringed by a cluster of microglia and cozied up to a blood vessel (see image above). Staining of tau revealed that Aβ and tau pathology often turned up in the same place. They also applied this technique to brains from aged Tg2576 and 3xTg mice, finding stark differences in the location of plaques in each mouse model. This technique may help people better decide which models to use and how to develop better ones in the future, they said.
They then applied iDISCO to blocks of frozen human hippocampal tissue. Labeled with PAB #10 for amyloid, the researchers were surprised to find large three-dimensional amyloid structures they called three-dimensional amyloid patterns, or TAPs. As big as 27 mm3, these plaque formations took many forms in different patients, such as thin sheets, densely packed blobs, parallel strips, and striated clumps (see examples below). Flajolet said he is not sure what these are, but wondered if their shapes could distinguish patients or correlate with specific disease symptoms. Perhaps they would help explain some of the variability seen in clinical trials, and provide a way to stratify patients in future studies, the authors wrote. That these could be detected in frozen human brain samples means TAPs could be studied in banked human tissue, said Flajolet.

Strange structures. In the brains of postmortem patients who died with Alzheimer’s, plaques congregate into unusual patterns. [Cell Reports, Liebmann et al.]
“The spatial organization of plaques is better appreciated in three dimensions than in the conventional two-dimensional histological section,” wrote Nigel Cairns, Washington University in St. Louis School of Medicine, to Alzforum (see full comment below). Such imaging will help understand how plaques develop over time, and relate to blood vessels, microglia, tau pathology, and neurodegeneration. Scientists may need to develop more suitable antibodies that better penetrate tissue, he noted. He said that the three-dimensional nature of plaques was already well known, so he was not surprised by the formations seen in human tissue.
“This is an impressive and novel way to image Aβ deposits that provides views of the diverse morphologies of Aβ deposits we have not seen before,” said John Trojanowski, University of Pennsylvania School of Medicine, Philadelphia. “It will be interesting to learn if further use of these methods provides us with new understanding or insights into mechanisms of Aβ deposition and neurodegeneration in AD.”
iDISCO will help image not only plaques, but also neuronal connectivity and neuron-glia relations, granting insights on brain structure, function, and disease pathogenesis, wrote Dietmar Thal, KU-Leuven, Belgium, to Alzforum (see full comment below). He added that the TAPs observed here resemble diffuse plaques already described, such as lake-like amyloid deposits in the presubiculum or fleecy amyloid in the entorhinal cortex (Wisniewski et al., 1998; Thal et al., 1999). He noted that classical immunohistochemistry can address most research questions with high resolution, so three-dimensional clearing methods should be reserved for questions that require the third dimension.—Gwyneth Dickey Zakaib
Media
References
News Citations
- Brain Anatomy Revealed With CLARITY
- With ScaleS, You Can See Through the Brain to Behold Synapses
- Mice Tell Tale of Tau Transmission, Alzheimer’s Progression
- Tau’s Synaptic Hats: Regulating Activity, Disrupting Communication
- Deadly Delivery: Microglia May Traffic Tau Via Exosomes
Research Models Citations
Mutations Citations
Antibody Citations
Paper Citations
- Wisniewski HM, Sadowski M, Jakubowska-Sadowska K, Tarnawski M, Wegiel J. Diffuse, lake-like amyloid-beta deposits in the parvopyramidal layer of the presubiculum in Alzheimer disease. J Neuropathol Exp Neurol. 1998 Jul;57(7):674-83. PubMed.
- Thal DR, Sassin I, Schultz C, Haass C, Braak E, Braak H. Fleecy amyloid deposits in the internal layers of the human entorhinal cortex are comprised of N-terminal truncated fragments of Abeta. J Neuropathol Exp Neurol. 1999 Feb;58(2):210-6. PubMed.
Other Citations
Further Reading
Papers
- Azaripour A, Lagerweij T, Scharfbillig C, Jadczak AE, Willershausen B, Van Noorden CJ. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog Histochem Cytochem. 2016 Apr 14; PubMed.
Primary Papers
- Liebmann T, Renier N, Bettayeb K, Greengard P, Tessier-Lavigne M, Flajolet M. Three-Dimensional Study of Alzheimer's Disease Hallmarks Using the iDISCO Clearing Method. Cell Rep. 2016 Jul 26;16(4):1138-52. Epub 2016 Jul 14 PubMed.
- Fu H, Hussaini SA, Wegmann S, Profaci C, Daniels JD, Herman M, Emrani S, Figueroa HY, Hyman BT, Davies P, Duff KE. 3D Visualization of the Temporal and Spatial Spread of Tau Pathology Reveals Extensive Sites of Tau Accumulation Associated with Neuronal Loss and Recognition Memory Deficit in Aged Tau Transgenic Mice. PLoS One. 2016;11(7):e0159463. Epub 2016 Jul 28 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
A major unresolved question in the pathogenesis of Alzheimer's disease (AD) is the mechanism by which Aβ aggregates induce neurofibrillary degeneration in nerve cells and their processes. Microscopic studies reveal that the Aβ plaque, one of the signature lesions of AD, the other being the neurofibrillary tangle (NFT), is a complex extracellular structure composed of filamentous Aβ, abnormal nerve cell processes called dystrophic neurites, reactive astrocytes, and activated microglial cells. The glial cells may have both a beneficial effect by removing “bad proteins,” e.g., Aβ filaments, and a detrimental effect by releasing neuroinflammatory chemicals that cause neuronal stress and abnormal cell function. Visualizing these components of the Aβ plaque in three dimensions may help to elucidate the temporal contribution of each to the evolution of the plaque and subsequent neurodegeneration. Thus, this paper by Liebmann and colleagues at Rockefeller University will be of great interest to students of AD.
To facilitate visualization of the intrinsic structure of the mouse brain and of small volumes of human postmortem brain, Liebmann and colleagues have applied an established method to make the brain more translucent called immunolabelling-enabled three-dimensional imaging of solvent cleared organs (iDISCO). The advantages of this method are several and include: rapid staining of tissue, automated whole (mouse) brain imaging, atlas alignment (e.g., Allen Brain Atlas), confocal microscopy, and automated object quantification (e.g., number, volume, inter-object distance). These methods are likely to engender more complex, but more realistic, modeling of plaque formation in both animal models of AD and postmortem brain.
The authors show what may be achieved using iDISCO in the study of AD. The spatial organization of plaques is better appreciated in three dimensions than in the conventional two-dimensional histological section. The relationship between one molecular pathology, the Aβ plaque, and the local neuroanatomy can be readily appreciated using antibodies to markers of blood vessels and glial cells, for example. Similarly, other molecular pathologies, such as tauopathy, the other molecular pathology of AD, can be visualized in a similar manner, which will allow for more sophisticated spatial studies that are likely to throw light on the pathogenesis of AD.
As with any new technology, there are potential pitfalls. iDISCO is most suitable for small molecular probes, such as the dye Congo red, and less suitable for large ones such as antibodies, which have a harder time penetrating the matrix supporting the tissue. This is a concern because antibodies are typically more sensitive than histological stains such as Congo red. In the Liebmann study, of 11 anti-Aβ antibodies, only one was sufficiently sensitive and penetrated the whole mouse brain and only 1 cm in human postmortem brain. As few well-characterized antibodies are used currently in the routine histology laboratory, new antibodies may need to be developed that are compatible with iDISCO. The software used to visualize microstructures could be improved. For example, the segmentation of the amyloid plaques generated globular structures where both immunohistochemistry and fine structural studies reveal that the Aβ plaque contains filamentous structures.
Since the original descriptions of senile plaques, pathologists have known that this is a three-dimensional structure and has different morphologies depending on the plane of section. The introduction of a new acronym, the three-dimensional amyloid pathology (TAP), to describe this well-characterized morphology is not illuminating, whereas iDISCO has every possibility of generating new insights into plaque formation. In summary, iDISCO has both advantages and some pitfalls in characterizing the neuroanatomy of the signature lesions of AD. Neuroscientists and neuropathologists will need to balance these competing factors in determining how best their research goals may be met with iDISCO.
Katholieke Universiteit Leuven, Department of Imaging and Pathology, Laboratory of Neuropathology
A third dimension of brain research?
Liebmann et al. used the iDISCO clearing methods to stain amyloid plaques immunohistochemically as well as with staining dyes (Congo red, thioflavin s) to assess their three-dimensional structure. Compared to the classical approach of three-dimensional reconstruction using serial sections, the iDISCO method is less laborious and, thus, permits the analysis of three-dimensional relationships in more detail and with higher numbers of cases.
In their published study, Liebmann et al. tested several staining methods for their feasibility in cleared tissue and could show that immunohistochemistry works well in this approach. The use of immunohistochemistry makes this clearing technique attractive for all kind of morphological studies aiming at the description of three-dimensional relations, especially for studying not only plaques but also neuronal connectivity, neuron-glia relations, etc. Such studies will produce novel insights into brain structure and function or disease pathogenesis constituting a novel level (“the third dimension”) of neuroscience.
When comparing plaques in the 2xtg AD-transgenic mouse brain with those in the human brain they could show that human plaques are more complex and larger than those in 2xtg AD-transgenic mice. This finding fits well with the well-known complex architectures of diffuse plaques that, for example, result in lake-like presubicular amyloid or in fleecy amyloid in the entorhinal cortex.
Thus, Liebmann et al. gave us a small but very promising look at what will be possible with the novel tissue-clearing methods.
However, when deciding between the use of classical immunohistochemistry and three-dimensional clearing approaches to address a research question, it is essential to keep in mind that classical immunohistochemistry will address more than 90 percent of the research questions, whereas three-dimensional clearing methods like iDISCO or CLARITY should be reserved for those questions that require the third dimension. Otherwise, normal immunohistochemistry provides a better cellular/subcellular resolution and may be more convincing, especially for double-labelling studies.
Mayo Clinic Florida
This is an elegant study by Drs. Duff and Hyman’s groups on the longitudinal characterization of the EC-tau mouse model. iDisco three-dimensional imaging of the whole translucent hemibrain is impressive and certainly quantitative at the mesoscale. Significant neuroinflammation is observed in the amygdala, which is an important region for aversive learning and can be tested by fear conditioning.
The authors distinguish cell autonomous/non-autonomous human tau (htau) accumulation based on in situ hybridization (ISH) of human tau mRNA. However, they observe hardly any htau mRNA signal in the MEC region (Fig. 5), which is where the htau transgene was originally designed to be expressed. If it is almost undetectable in the MEC, it will not be detectable anywhere else. Therefore, control ISH for mouse tau mRNA in the MEC region would be desirable to verify the ISH technique.
Because the authors found little evidence for tau accumulation in astrocytes and microglia in the mouse brains, they suggested these cells play no major role in the propagation of tau. However, the lack of accumulation of tau in microglia does not disprove their role in phagocytosis of neurons or pathogenic proteins. Immune cells, and microglia in particular, are not designed to store proteins intracellularly. After phagocytosis of exogenous materials, they digest them, then secrete for antigen presentation, or die if neither digestion nor secretion is effective. In addition, while the authors use IBA1 (or Cx3cr1-GFP) staining to detect microglia, P2ry12 is microglia-specific and is expressed on their extracellular regions, which extend much further in space than the cytoplasmic regions. Therefore, P2ry12 staining is better for visualizing extended and more-branched microglial terminals that might envelope cytopathic neurons accumulating tau or other toxic molecules.
Make a Comment
To make a comment you must login or register.