Excited Neurons Release More Aberrant Tau
Quick Links
The idea that pathological forms of proteins spread along neuronal circuits, corrupting normal proteins as they go, has gained traction among researchers studying neurodegenerative diseases. However, scientists are still puzzling out the mechanism of how misfolded proteins get from one neuron to the next, and whether this protein mobilization contributes to pathology. A new study suggests it does—at least in mice. Researchers led by Karen Duff, Columbia University, New York, report that tau spreads among cells when neurons expel the protein into the extracellular space. Further, they find that this depends on neural activity. More active neurons release more tau, and this leads to more neurofibrillary tangles forming in the hippocampus. Since Aβ is known to overly excite neurons, the authors interpret their results as evoking a link between this peptide and the propagation of neurofibrillary tangles. “Aβ might exacerbate tau pathology,” said Duff.

Taking up Tau. In microfluidic chambers, tau (red) migrates along axons to adjacent cambers to be taken up by tau knockout neurons (green). [Courtesy of Wu et al., Nature Neuroscience.]
Some studies suggest that neurons spit tau out into the extracellular space (Saman et al., 2011; Dujardin et al., 2014). Others have reported that the most active neurons release more tau (Feb 2014 news; Aug 2013 conference news; Pooler et al., 2013). However, whether this extracellular tau contributed to pathology remained unclear.
Initially, first author Jessica Wu tested whether human tau that leaked into the extracellular space was able to enter other cells. She and her colleagues cultured primary neurons from rTg4510 mice, which overexpress human tau containing the P301L frontotemporal dementia mutation, then used that conditioned medium to culture neurons from tau knockout or wild-type mice. They found that tau from the medium began to accumulate inside the cells. Medium from iPSC-derived neurons had the same effect. The results suggest that misfolded tau released into the extracellular space can pass between cells, without the aid of direct cell connections, such as tunneling nanotubes (see Rustom et al., 2004; Apr 2016 Alzforum webinar on pathogenic protein spread).
To test whether tau could spread between cells, the researchers cultured rTg4510 mouse neurons on one side of a two-chambered microfluidic device with tau knockout neurons on the other. The membrane separating the compartments allows axons to penetrate, but keeps the culture media from mixing. This ensures that any solutes added to the medium on one side do not diffuse into the other. After two weeks, tau could be seen building up in the tau knockout neurons, suggesting the protein had passed from one neuron to another (see image above).
To see if endogenous tau could be coaxed to misfold and then spread to other neurons, the researchers grew three separate populations of P301S tau mouse neurons in a three-part microfluidic chamber. They all expressed a version of tau labeled with yellow fluorescent protein. When the researchers added oligomers of misfolded tau to the first chamber, the fluorescently labeled endogenous tau began aggregating inside the neurons there, indicating the cells took these oligomers up, and that they caused healthy tau to misfold. What’s more, after 12 days clumps of fluorescent tau appeared in the neurons of the second chamber, and after 17 days in the third. This implies that seeds can aggregate tau in neurons, then propagate over long distances along axons. However, the authors have yet to work out how internalized tau finds its way to endogenous tau in the cytoplasm to template misfolding.
What about neuronal activity driving tau release? To test this, Wu and colleagues expressed light-driven channelrhodopsin ion channels in primary neurons from wild-type mice that express human tau. When they induced action potentials in these cells with flashes of blue light, tau in the media climbed 2.5-fold over 30 minutes. In a different experiment, stimulating cultured primary mouse neurons with picrotoxin led twice as many to take up tau from neighboring cells. Duff was not sure if the cells took up the tau more actively, or if there was just more released into the medium.
To test whether hyperactivity-driven tau release and uptake contributes to pathology, the researchers expressed the same light-sensitive channel in the forebrain of the rTg4510 mouse model. For 20 days they intermittently illuminated the left hippocampus, doubling its normal firing rate. Immunolabeling revealed that more tau accumulated, and more neurons died, in the left hippocampus than the right (see image below). In a similar, chemogenetic experiment, the authors expressed a designer receptor exclusively activated by a designer drug (DREADD) in the left entorhinal cortex of rTgTauEC mice, which express P301L human tau predominantly in the entorhinal cortex. Injecting the DREADD drug clozapine-n-oxide twice daily stimulated those neurons. Over six weeks, tau accumulated in the cell bodies and dendrites of the left entorhinal cortex, but not the right. “That leads us to believe that enhancing neuronal activity leads to greater pathology in vivo,” said Duff. Tau aggregates did not appear in the left dentate gyrus of the hippocampus, which receives input from the entorhinal cortex. Duff and colleagues had previously reported that tau pathology in this model spreads to the hippocampus (see Feb 2012 news). The authors noted that it would probably take more than the six weeks of this chemogenetic experiment for tau to turn up in the hippocampus.
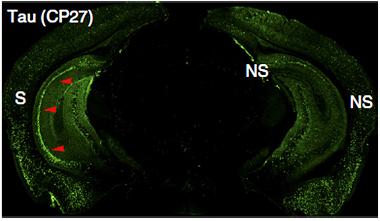
Lopsided Load.
Stimulated neuronal activity in the left (S) hippocampus of rTg4510 mice leads to a boost in tau pathology (green), compared to the unstimulated right side (NS). [Courtesy of Wu et al., Nature Neuroscience.]
Together, the results suggest that tau can be released to, and taken up from, the extracellular space and pass between cells, and that hyperactive neurons churn out the protein and worsen pathology.
Scientists have yet to determine if this type of propagation occurs in people and if it contributes to a decline in cognition. Precisely how neurons release tau is also still unclear, though recent work suggests that chaperon-mediated pathways rid cells of accumulating misfolded proteins, such as tau (see Jun 2016 news; Jun 2016 news).
“It is interesting that tau can spread through the extracellular space, providing a mechanism for local spread, and that this can be driven, to some extent, by hyperexcitability,” noted Aaron Schultz, Massachusetts General Hospital, Charlestown. “Given the association between amyloid and neuronal excitability, these findings potentially explain how amyloid pathology catalyzes tau pathology.” It will be interesting to see what regional patterns emerge in longitudinal tau imaging with positron emission tomography, as that should provide useful information about the spread of tau pathology across different disease states, he added.
If neuronal activity modulates tau release in people, it could have several implications, said Duff, not least being that patients undergoing deep brain or transcranial magnetic stimulation to boost their neural activity should potentially be monitored for tau levels. It could also support the idea that scientists might be able to prevent the spread of tau by targeting it in the extracellular space, Rik Ossenkoppele, VU University Amsterdam, wrote to Alzforum in an email. In addition, antiepileptic drugs such as levetiracetam could prevent or slow the propagation of tau by reducing hyperactivity of hippocampal neurons, Ossenkoppele said. A low-dose version of levetiracetam, called AGB101, will soon enter a Phase 3 trial in patients with amnestic MCI (see Nov 2015 news).—Gwyneth Dickey Zakaib
References
News Citations
- Neurons Release Tau in Response to Excitation
- Tales of Traveling Tau: Is Transfer Between Neurons Normal?
- Mice Tell Tale of Tau Transmission, Alzheimer’s Progression
- Can’t Degrade That Pesky Misfolded Protein? Push It Off the MAPS
- Ushers of Propagation? More Evidence that Chaperones Evict Disease-Associated Proteins
- Truly New to Déjà Vu: Investigational Therapy News at CTAD
Research Models Citations
Webinar Citations
Therapeutics Citations
Paper Citations
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid (CSF) in early Alzheimer's Disease. J Biol Chem. 2011 Nov 4; PubMed.
- Dujardin S, Bégard S, Caillierez R, Lachaud C, Delattre L, Carrier S, Loyens A, Galas MC, Bousset L, Melki R, Aurégan G, Hantraye P, Brouillet E, Buée L, Colin M. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One. 2014;9(6):e100760. Epub 2014 Jun 27 PubMed.
- Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013 Apr;14(4):389-94. PubMed.
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004 Feb 13;303(5660):1007-10. PubMed.
Further Reading
News
- Deadly Delivery: Microglia May Traffic Tau Via Exosomes
- New Imaging Data Tells Story of Travelling Tau
- Propagation Blues? Reporter Expression Clouds Reports of Traveling Tau, Aβ
- Protein Propagation Real, but Mechanisms Hazy
- Is Dendritic Tau to Blame for AD-Related Hyperexcitability?
- Antibodies Stop Toxic Tau in Its Extracellular Tracks
Primary Papers
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016 Aug;19(8):1085-92. Epub 2016 Jun 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
VU University Medical Center
Using elegant optogenetic and chemogenetic approaches, the authors show that pathological strains of tau are transferred cell-to-cell via the extracellular space, and that this propagation can be observed in “recipient” neurons that are more than one degree of connection removed from “donor” neurons that are seeded with a fibrillar tau strain. They also show that increased neuronal activity in hippocampus is associated with higher levels of extracellular tau, more rapid spread of tau to recipient neurons, and more rapid hippocampal atrophy in tau transgenic mice.
The notion that pathogenic proteins in various neurodegenerative conditions do not spread in a random fashion, but rather follow a prototypical pattern that spatially overlaps with disease-specific functional networks, has given rise to a “network model of neurodegeneration.” This evidence-based theory offers several mechanisms that may explain this orchestrated spread of disease, including “shared vulnerability” (i.e., neural populations with similar genetic and/or molecular properties may be equally susceptible), “trophic failure” (i.e., disruptions along functional pathways hinder transport of trophic sources), and “transneuronal spread” (i.e., proteins propagate along network connections). The latter has been investigated extensively lately, and seeding studies have shown that functional networks may indeed serve as a template to predict the spatial trajectory of toxic agents. These fundamental studies have been translated into human neuroimaging experiments (using functional MRI [fMRI] and diffusion tensor imaging [DTI]), which consistently showed that the functional architecture of the healthy brain dictates where pathology will spread, starting from a disease-specific epicenter.
Both basic science and human neuroimaging studies have thus provided evidence for the trans-synaptic spread hypothesis. It is still under debate, however, whether this cell-to-cell transmission occurs through actual physical connections or via the extracellular space. This study is important as it provides evidence for the latter — perhaps least intuitive — mechanism, and additionally showed that increased neural activity might facilitate the release and propagation of tau. Both findings have potential ramifications for disease-modifying therapies aiming to prevent spreading of the disease, as one might speculate that 1) it would be more feasible to target tau species in extracellular space compared to when it is embedded intracellularly, and 2) anti-epileptic drugs (e.g., levetiracetam) could prevent or slow the propagation of tau by reducing (hyper)activity of hippocampal neurons.
While reading this paper, it became clear to me that there is much room for improvement in terms of communication between researchers who are investigating the mechanistic properties of neurodegenerative diseases from a cellular/molecular perspective in animal models and in vitro cultures vs. scientists in the imaging community who are looking to find evidence for these mechanisms operating on a much larger scale in living humans. It is obvious that some debates at the cell level cannot be broached by human imaging approaches (e.g., whether tau spreads extracellularly or via direct connections), but it is very important to identify questions that human imaging can weigh in on. For example, a key finding in this study by Wu et al. that brain regions with higher neuronal activity might, once exposed to tau pathology, aggregate tau faster and spread it to other regions more rapidly than regions with lower activity, sounds very testable in the living human brain using imaging techniques such as tau PET and fMRI.
Make a Comment
To make a comment you must login or register.