In the Finnish city of Kuopio, neurosurgeons collaborate with neurophysiologists and molecular and cellular biologists to make frontal cortex, dura, intraventricular CSF, skin, fat, and other tissue from hydrocephalus patients available for research purposes. Being in their 60s to 80s, half of these patients have preclinical ADRD pathology; some carry ADRD gene variants. The Kuopio biopsy sampling protocol improves patient care and fuels research studies in Europe and the U.S.
Series
Kuopio Hydrocephalus Shunt Biopsy Protocol
Fresh Brain Every Friday: Biopsies Transform Alzheimer's Science
Heads up, Alzheimerologists around the world. This serialized report from Kuopio, a small Finnish city close to the Russian border, is well worth considering. It is a story about how tight—sometimes minute-by-minute—integration between medicine and science can benefit patients suffering from an obscure illness while simultaneously propelling neurodegeneration research to the next level. Together, Ville Leinonen, Tarja Malm, and Mikko Hiltunen—faculty in different disciplines at the University of Eastern Finland—have pioneered a system that might well inspire other medical research centers around the world. (Bicycles are involved.)

Where It Happens. Kuopio, in southeastern Finland, site of a unique brain biopsy cohort. [https://commons.wikimedia.org/wiki/File:Kuopio_aerial_6.jpg]
In this remote lakeside town ringed by birch forests, what happens to a person who can't walk anymore, wears diapers, and feels his or her mind fading because age-related hydrocephalus squashes their brain tissue? He or she can undergo a day surgery at Kuopio University Hospital that will restore their quality of life. During the procedure, a brain surgeon places a catheter through the frontal cortex at the top of the brain into the right ventricle. From there the catheter will drain the troublesome excess CSF into their abdominal cavity (image below).

The Basic Idea. One end of a silicon catheter is pushed through a burr hole in the skull above the right frontal cortex, and into the brain's ventricle. The other end is threaded under the skin to the abdominal cavity. This drains excess CSF from the brain, relieving the symptoms of chronic hydrocephalus. [Courtesy of Ville Leinonen.]
This procedure happens in many operating rooms around the world. But only in Kuopio does the surgeon collect up to eight different tissue samples along the way, including frontal cortex. If you count blood and CSF draws during the patient's pre-op and follow-up visits, the number of tissue samples from a single person exceeds a dozen. Many of those serve diagnostic purposes to better the patient's care, but they are also deposited in Finland's tissue banks for research. One—a bit of cortex—is studied within an hour of the surgery itself.
Together, the tissues and clinical-cognitive data collected from these patients enable discoveries across a range of different scientific methodologies, and from many organs, in hundreds of deeply phenotyped longitudinal study participants. Because idiopathic normal-pressure hydrocephalus (iNPH) afflicts people in their 60s, 70s, and 80s, these participants represent the spectrum of presymptomatic AD neuropathology. Indeed, about half have only NPH, others have amyloid deposits, yet others have plaques and tangles, or other causes of dementia. In short, the participants of this interwoven surgery-research program provide a natural model of early stage Alzheimer's disease and related disorders (ADRD).
Elsewhere in the world, brain biopsies are occasionally available to Alzheimer’s scientists at various research centers, typically when surgeons resect tumors or areas originating epileptic seizures. But it's not the same. In those diseases, surgeons avoid removing tissue that is functionally sound. Each biopsy tends to come from a different region, often from adults too young to be most informative for Alzheimer's research, and with varying amounts of attendant phenotypic information. In Kuopio, cerebro-ventricular biopsy collection is as standardized as the shunt surgery procedure itself. The cerebral biopsies all come from the same gyrus in area Brodmann area 8 of the right frontal cortex. Every week, the same team of neurosurgeons collects, studies, and preserves the samples in exactly the same way.
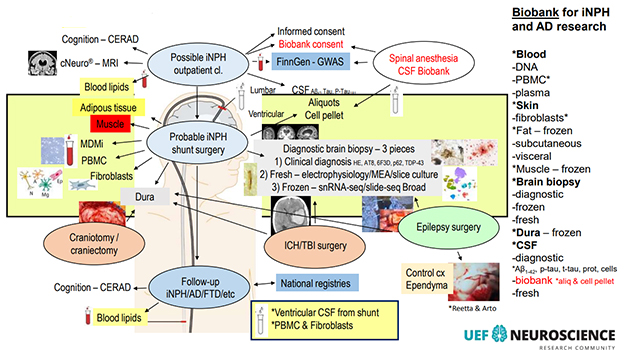
Lots Going On. This busy diagram lists most of the procedures in, and some research uses of, the Kuopio iNPH shunt sampling protocol. [Courtesy of V. Leinonen, KUH.]
One of the samples is unique in the world. It is a pyramid-shaped piece gently lifted from the top of the cortex where the shunt catheter will enter, and it is studied on the same day while still “alive.” No other lab does this. At 3 mm per side, the pyramid is only as wide as the silicon tube—the shunt—that will go into the cortex in its place. That is tiny compared to the occasional epilepsy or tumor resection biopsy a lucky lab scientist may get their hands on. Even so, this pyramid is big enough to encompass a "working unit" of cerebral cortex (image below). From a square of pia mater at its top, it reaches down through layers 1 to 6, to a tip of white matter at its bottom (see Part 2 of this series.)
Within five minutes of coming out of the patient, the pyramid leaves the operating room, lands in the hands of an electrophysiologist waiting by the hospital door, and rides by bicycle to a research building 0.8 miles down the hill. There, serial slices cut from it soon find themselves mounted in two recording rigs—one a single-cell patch clamp, the other a multi-electrode array. For the next eight hours or so, neurons in these acute human brain slices fire, burst, and oscillate—all while their donor up on the hill comes to in the recovery room, and gets ready to go home (for details, see Part 3 of this series).

From Brain to Vibratome in a Hurry. Within an hour of coming out, a pyramid-shaped piece of frontal cortex is sliced, recorded from, and preserved for other methods of study. [Courtesy of Henna Jäntti].
All tissues collected during these weekly ventriculo-peritoneal shunt placements form part of the most comprehensive, collaborative ADRD biopsy research protocol in the world. Beyond the cortical pyramid, it has many additional components.
For example, the neurosurgeon, with a punch needle, pulls two even tinier bits of cortex from where the pyramid just came out and slides them into liquid nitrogen. They are shipped to the labs of Evan Macosko and Beth Stevens at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. There, scientists profile the gene expression signatures of each cortical cell type and run multi-omics studies feasible only with fresh frozen brain.
A first major manuscript from this ongoing work by the Broad/UEF collaborators appeared on June 5 on bioRXiv. It characterizes, in the brains of living people, early cellular changes across three stages of increasing AD pathology. In a nutshell, the loss of a particular type of layer 1 inhibitory neuron appears to render a particular type of layer 2/3 excitatory neuron hyperactive. These neurons fade soon after, while microglial subpopulations shift toward neuroinflammatory states. The Aβ instigating this slow-moving calamity comes not only from the neurons themselves, but also from oligodendrocytes and their interface with axons of the doomed pyramidal neurons, drawing attention to myelin in early AD (Gazestani et al., 2023; Part 4 of this series).
The Broad also receives blood from the same patients for whole-genome sequencing. Other vials of blood stay at the University of Eastern Finland. One is used for the patient's diagnosis and follow-up care; one goes to Hiltunen's lab for studies exploring its monocytes; others are frozen and banked for proteomic, metabolomic, and lipidomic analyses with collaborators at UEF and elsewhere.
A sliver of skin is taken—not separately via punch biopsy as is customary in research, but right as the surgeon opens the abdomen to situate the end of the shunt catheter descending, underneath the patient's skin, from his or her brain. This skin sample will yield fibroblasts that are reprogrammed to iPSCs, then differentiated into various cell types for research use. This happens in Malm's lab at UEF's A.I. Virtanen Institute for Molecular Sciences.
Four other types of tissue from the patients have been banked at UEF for some years. Biding their time in a freezer, they are awaiting the right research question to emerge for projects either at UEF or in collaboration with other universities. They are: lumps of fat from under the skin; if present, lumps of fat from the peritoneal cavity near where the catheter ends; a morsel of muscle from the abdominal wall; and slivers of dura for research on the brain's border tissues.
CSF is taken often, and copiously. After all, having too much of it is part of the problem for people with NPH. When a person whose symptoms could be due to NPH gets referred to Kuopio University Hospital, a spinal tap drawing 40 ml is part of the diagnostic workup. That's because if the patient’s gait improves after such a large reduction in CSF volume, it is a hint NPH may be the underlying etiology. Adult humans have, on average, 120 to 150 ml of CSF. Confirmation of the NPH diagnosis requires a subsequent MRI.
During the shunt surgery, nurses collect up to 20 ml of CSF directly from the right ventricle the moment the shunt tip enters. This relieves pressure in the patient's brain, accelerating symptom relief. It also gives researchers an opportunity to compare the CSF deep inside a person's brain with CSF in the spinal canal. One such study quantified the concordance of ventricular and lumbar CSF Aβ42, total tau, and p-tau 181 in 138 NPH patients (Lukkarinen et al., 2023).
The Kuopio protocol calls for the surgeon and her nurses to collect the first few drops coming out of the freshly shunted ventricle in a separate tube from the bulk of the ventricular fluid volume. Why? This initial drip contains cortical cells shorn off as the shunt catheter penetrates through the gyrus into the ventricle. These cells are spun down and cryoprotected, so that their transcriptomes can be studied separately from those of the immune cells more typically found in CSF.
What's in it for the patient?
The data being generated from this range of tissues exist in a rich context thanks to detailed phenotypic and clinical information being gathered on each participant. After the surgery, patients return to the clinic for many follow-up visits, both to improve their care and for research. At the first post-op, doctors optimize the shunt's flow via a small valve implanted under the skin behind the ear. Later visits are annual checkups if the shunt works fine, or “repair” visits should it get blocked. Each time, if malfunction is suspected, a minor skin puncture to access the valve generates yet another sample of ventricular fluid for longitudinal research. Most of it gets banked at UEF; aliquots go to Sweden's University of Gothenburg as part of a long-standing proteomics and biomarker collaboration with Kaj Blennow and Henrik Zetterberg.
The cortex pyramid, too, serves multiple purposes beyond satisfying the curiosity of electrophysiologists. All but the two serial slices that are used in the live recordings are prepped for other uses. One gets frozen for Slide-Seq spatial transcriptomics of the intact cellular architecture of aging human cortex. One gets fixed and sliced into thin sections for research immunohistochemistry, and one is for electron microcopy. Any extra bits go toward proteomic studies. Those are research uses.
One slice of the pyramid goes back up the hill to the university hospital, where neuropathologist Tuomas Rauramaa stains them for routine clinical diagnostic markers. Using antibodies against pathological forms of Aβ, tau, α-synuclein, TDP-43, as well as markers of Trem2/microglial activation and the autophagy marker p62, Rauramaa's team generates molecular information to sharpen the shunt recipient's diagnosis. Knowing whether a patient has “pure” hydrocephalus, or also a concomitant neurodegenerative process, informs their follow-up care.
In Kuopio, diagnostic use of biopsies in longitudinally followed shunt patients started more than 30 years ago, hence scientists today know more about how NPH overlaps with age-related neurodegenerative diseases. For example, amyloid positivity is more frequent among people with NPH than in healthy controls, perhaps because an underlying drainage problem accelerates accumulation of Aβ (e.g., Luikku et al., 2019). Even so, most patients' NPH symptoms improve for a while after the shunt procedure, regardless of whether they have plaques, or plaques and tangles. For this reason, all patients with a confirmed NPH diagnosis are candidates for shunt surgery in Kuopio, even those with concomitant neurodegenerative disease. Typically, the latter benefit for fewer years than those with “pure” NPH, as the shunt does not treat the other illnesses. The mean survival time after shunt placement is five years, according to Leinonen.
How Much Does the Operation Help?
Up to 90 percent of shunt recipients can cast aside their walker or wheelchair, and regain control of their bladder, Leinonen told Alzforum. Regarding cognition, people with MCI before surgery tend to revert to normal; those with mild dementia improve to MCI and regain independence. Surgeons at five centers across the U.S. confirmed that this treatment is safe and effective (Williams et al., 2022), and a Swedish study found that it prolongs life (Andrén et al., 2021). A multicenter RCT is ongoing in the U.S.
But it is not a permanent solution. One study reported that only a third of recipients are still better by three years post-op (Espay et al., 2017). To this cautionary finding, the Kuopio and Gothenburg teams responded by searching for biomarkers that might predict shunt response. On June 5, they report that an unbiased tandem mass tag proteomic analysis of 68 shunt recipients' pre-operative CSF netted nine candidate markers (Weiner et al., 2023).
A person's longer-term cognitive outcome depends on whether (s)he has another neurodegenerative disease. Appearances, however, can be deceiving. For example, Leinonen recalled a man whose MMSE had risen post-shunt to 29/30, but over the next 2.5 years gradually sank again, to 19. "He had amyloid in his biopsy, so we thought now it may be the AD taking over," Leinonen told Alzforum. "But we checked his shunt and found it was blocked. After that was cleared, his MMSE went back up to 26 after two months, and he functioned quite normally again." This underscores the importance of follow-up care: "When our patients worsen, we often can get them back on track," he said (image below).
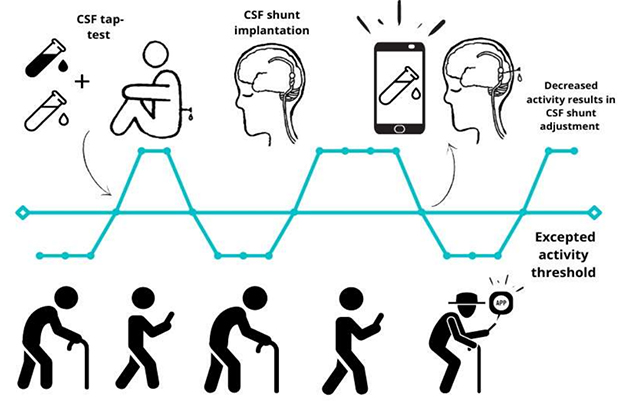
Track That Shunt. During follow-up care, adjustments to the shunt's flow can restore its treatment benefit, while also generating longitudinal CSF samples. In the future, digital monitoring may detect when the shunt malfunctions. [Courtesy of Ville Leinonen and Rosa Sahlström, KUH.]
For the treating physicians, having data on both cortical tissue and CSF paints a more complete picture of the patient in front of them. Ditto for their likely prognosis. Alas, partly because NPH often overlaps with other diseases of aging, NPH is not treated aggressively in many clinics around the world. Without a molecular diagnosis, this murkiness can feed nihilism among neurologists, who may think: "Why subject this person to surgery when they probably have some untreatable dementia?" In contrast, the Kuopio clinician-researchers see this overlap as an opportunity: "We can make this person better while studying both their NPH and their preclinical Alzheimer's or FTD," Leinonen said.
The risk of overtreatment is small, he believes, because MRI easily rules out hydrocephalus. The condition grossly distends the ventricles, creating dramatic, fluid-filled caverns where brain tissue should be. NPH also flattens the distinctive folds of gyri and sulci in areas where the cortex gets pressed against the skull (image below).

Too Much Pressure. MRI scans of chronic hydrocephalus. The disease enlarges the brain's ventricles, creates CSF-filled cavities, and can flatten cortical gyri. The right image represents a subgroup called LOVA. [Courtesy V. Leinonen].
The diagnostic benefit of the brain and CSF biopsies explains why the Kuopio sampling protocol comports with medical ethics regulations. In Finland, these are partially based on the Declaration of Helsinki and are as rigorous as in any nation in the world. Because diagnostic biopsies are an integral part of patient care, a generic “umbrella” consent for their additional research use suffices to support this highly differentiated hydrocephalus shunt protocol. The sampling does not increase patients' risk during surgery, unlike trials of experimental therapies, which do come with new and unknown risks.
Most patients consent readily. "Finnish people are positively inclined toward research," Leinonen told Alzforum. In the rare cases when a patient declines research use of their shunt biopsies, the scientists take no skin, fat, or muscle, and a smaller volume of blood. They still use a cortical biopsy and CSF for diagnosis, but do not bank it.
Surprisingly, perhaps, removing a small volume of cortical tissue causes no known cognitive loss. In humans, no specific function has yet been discovered for the gyrus in question. That is why Brodmann 8 serves as a standard point of entry when trauma neurosurgeons have to “go in” to lower intracranial pressure, or drain fluid, in emergency situations, such as brain trauma after an accident, or subarachnoid hemorrhage after a stroke or aneurysm.
Who set up this sampling protocol? The lead instigator is Leinonen, a neurosurgeon at UEF. A spinal neurosurgeon by specialty, Leinonen says he thinks about research all the time. His interest was kindled when, as a resident and postdoc, he used hydrocephalus frontal cortex biopsy tissue to validate a then-brand-new amyloid PET tracer called PIB (Leinonen et al., 2008). "That was my first experience with Alzheimer's research," he said.

Doing Well While Doing Good With Biopsies? Ville Leinonen at Kuopio University Hospital, holding a Dewar that will snap freeze bits of cortex and dura the minute they are taken from the human brain, for RNA-Seq and other analyses at the Broad Institute in Cambridge, Massachusetts. [Image courtesy of Gabrielle Strobel.]
A key partner is Malm. A molecular biologist, she runs a 25-person lab. Every week for six months in 2020, Antonios Dougalis, a neurophysiologist in Malm's lab, conferred with Leinonen's team of neurosurgeons as he tried to establish recordings from the biopsies. Dougalis realized that conventional ways of sampling brain tissue—sucking out a worm-shaped piece with a syringe needle or grabbing a bit with biopsy forceps—squeezed the neurons inside such that they would not fire. This prompted Antti Luikku, a resident with Leinonen, to cut four pyramid sides with the tip of a surgery knife and lift it out ever so delicately on the knife's flat side. Other scientists in Malm's lab are making iPSC-derived cell types and organoids from skin biopsies of interest, particularly from shunt patients who happen to carry genetic variants sought after for mechanistic studies, such as in progranulin and other genes.
Last but not least, the whole operation is part of UEF's larger Neuroscience Research Community, an organizational and funding framework overseen by Hiltunen, a molecular geneticist.
In 2013, Hiltunen and Leinonen began extracting RNA from iNPH cortex biopsies. They learned then that they needed to flash-freeze the tissue right in the operating room if they wanted the RNA to stay intact for sequencing. From there, Hiltunen and Leinonen continued building a sampling program. Today, Mari Takalo in Hiltunen's lab runs research projects that use NPH biopsy tissues for functional genomics and personalized medicine studies. She currently focuses on variants in PLCG2, progranulin, Abi3, APP, TREM2, TyroBP—all of which occur among Kuopio's NPH shunt recipients. Scientists led by UEF molecular biologist Annakaisa Haapasalo study C9ORF72. Hexanucleotide expansions in this gene are Finland's most common cause of FTD—and are found in the NPH shunt cohort (for more, see Part 5 of this series).
A Look Back in Time
The Kuopio biopsy research protocol evolved over the past three decades. At KUH, the practice of improving hydrocephalus shunt placement with biopsies dates back to 1991, when the late neurosurgeon Matti Vapalahti trained his resident Sakari Savolainen, who made the combination routine. An early champion through the 1990s and 2000s was Irina Alafuzoff, a prominent ADRD neuropathologist who worked in Kuopio before moving to Sweden's Uppsala University. Another was neurosurgeon Juha Jääskeläinen, who forged the connection between NPH and AD research when Leinonen was a resident, starting in 2005.
In 2010, the KUH neurologist Anne Koivisto explored the link between NPH and AD. She retrospectively analyzed brain biopsies stained for Aβ and tau going back 20 years. Koivisto screened intake records from 468 NPH patients to match cognitive decline noted in them against diagnostic neuropathology criteria for AD as seen in their cortices. This led to the realization that nearly half of shunted NPH patients—including those who after the shunt get better for several years—develop dementia eventually. Mostly, they were the ones who had AD pathology, followed by those with vascular pathology (Leinonen et al., 2010). In the 13 years since, postmortem neuropathology findings of those early patients, plus biopsy data from more recent ones, have made clear that about one in 100 people with NPH also have multiple-system atrophy, Lewy body dementia, corticobasal degeneration, or another frontotemporal dementia, Leinonen told Alzforum.
The older components of the Kuopio NPH biopsy protocol—cortex, CSF, CSF cells, blood, skin—have been banked for many years such that samples and cell lines are available on several hundred patients. Over the years, the protocol has become standardized.
It also has grown, both in terms of how many shunts are placed each year, and how much information is being collected on each patient. Since acute slice electrophysiology started in 2020, preoperative fMRI was added in June 2022. Currently, scientists are working out how to also collect a tiny drop of CSF from the arachnoid space right above the brain in hopes of enabling research on these border layers. Also under consideration: adding preoperative EEG to relate the results of acute cortical slice recordings, i.e., local activity, with regional brain activity.
Nowadays, 50 NPH shunts are placed at KUH annually by a team of brain surgeons. It is a routine procedure, performed by residents on most Fridays and some Tuesdays. NPH shunt placements in the country's other four university hospitals—Helsinki, Oulu, Tampere, and Turku—bring the annual number in all of Finland to 150, according to Leinonen, though not all sites collect research biopsies comprehensively.
Some other centers in Scandinavian countries take and study NPH shunt biopsies. Research on NPH itself occurs at Rikshospitalet in Oslo, most recently with a study visualizing the fragile meningeal lymphatic vessels in dura mater (Vera Quesada et al., 2023). ADRD fluid-based biomarker and proteomics science with NPH CSF happens at UGothenburg, and tissue neuropathology research at University of Uppsala, from where Alafuzoff recently retired. Throughout Europe and the U.S., shunts are available as a treatment for hydrocephalus, but these sites do not routinely collect and bank biopsy tissue as part of an ongoing ADRD clinical research program.
Some one-off studies exist. For example, in New York City, Columbia University neurosurgeon Guy McKhann and neuropathologist Andrew Teich published bulk RNA-Seq analysis of NPH cortical biopsies, described gene expression patterns indicating a microglial state change when there was concomitant AD pathology (Huang et al., 2021). At Johns Hopkins University in Baltimore, researchers used NPH shunt biopsies to validate the amyloid PET tracer flutemetamol (Wong et al., 2013).
Leinonen and colleagues contribute their data on the cohort of NPH shunt patients—more than 1,000 going back to 1993—to FinnGen, the country's equivalent of the U.K. Biobank, for DNA genotyping and data integration. FinnGen serves as the national hub for de-identified clinical data on the NPH patients' clinic visits, hospitalizations, surgeries, prescriptions, and causes of death for those who have died. The FinnGen dataset amounts to searchable lifelines of these people alongside similar data on soon-to-be 500,000 other Finns as controls (see Part 5).
The Kuopio researchers collaborate on these biopsy projects with colleagues in Norway, Sweden, Germany, and the U.S. If more hospitals that place those shunts built similar tissue collection protocols, studies could combine forces to achieve larger group sizes, especially on the biology of rare gene variants. Age-related hydrocephalus is, after all, itself a relatively rare disease. Leinonen told Alzforum that the Kuopio team would be happy to teach visiting surgeons and neurophysiologists interested in starting similar programs at their universities. (Parts 2, 3, 4, 5).—Gabrielle Strobel
References
News Citations
- A Day's Work: Cortex Biopsy Comes Out. Shunt Goes In. Patient Goes Home.
- Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
- Cortical Biopsies Hint at Start of Alzheimer's 'Cellular Phase'
- Brain Biopsies and FinnGen Form Wellspring for Functional Genomics
Paper Citations
- Gazestani VH, Kamath T, Nadaf NM, Burris S, Rooney B, Junkkari A, Vanderburg C, Rauramaa T, Therrien M, Tegtmeyer M, Herukka S-K, Abdulraouf A, Marsh S, Malm T, Hiltunen M, Nehme R, Stevens B, Leinonen V, Macosko EZ. Early Alzheimer's disease pathology in human cortex is associated with a transient phase of distinct cell states. 2023 Jun 05 10.1101/2023.06.03.543569 (version 1) bioRxiv.
- Lukkarinen H, Vanninen A, Tesseur I, Pemberton D, Van Der Ark P, Kokkola T, Herukka SK, Rauramaa T, Hiltunen M, Blennow K, Zetterberg H, Leinonen V. Concordance of Alzheimer's Disease-Related Biomarkers Between Intraventricular and Lumbar Cerebrospinal Fluid in Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2023;91(1):305-319. PubMed.
- Luikku AJ, Hall A, Nerg O, Koivisto AM, Hiltunen M, Helisalmi S, Herukka SK, Junkkari A, Sutela A, Kojoukhova M, Korhonen V, Mattila J, Lötjönen J, Rummukainen J, Alafuzoff I, Jääskeläinen JE, Remes AM, Solomon A, Kivipelto M, Soininen H, Rauramaa T, Leinonen V. Predicting Development of Alzheimer's Disease in Patients with Shunted Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2019;71(4):1233-1243. PubMed.
- Williams MA, Nagel SJ, Golomb J, Jensen H, Dasher NA, Holubkov R, Edwards RJ, Luciano MG, Zwimpfer TJ, Katzen H, Moghekar A, Wisoff JH, McKhann GM, Hamilton MG. Safety and effectiveness of the assessment and treatment of idiopathic normal pressure hydrocephalus in the Adult Hydrocephalus Clinical Research Network. J Neurosurg. 2022 Mar 11;:1-13. PubMed.
- Andrén K, Wikkelsø C, Hellström P, Tullberg M, Jaraj D. Early shunt surgery improves survival in idiopathic normal pressure hydrocephalus. Eur J Neurol. 2021 Apr;28(4):1153-1159. Epub 2021 Jan 25 PubMed.
- Espay AJ, Da Prat GA, Dwivedi AK, Rodriguez-Porcel F, Vaughan JE, Rosso M, Devoto JL, Duker AP, Masellis M, Smith CD, Mandybur GT, Merola A, Lang AE. Deconstructing normal pressure hydrocephalus: Ventriculomegaly as early sign of neurodegeneration. Ann Neurol. 2017 Oct;82(4):503-513. Epub 2017 Oct 4 PubMed.
- Weiner S, Junkkari A, Sauer M, Luikku A, Rauramaa T, Kokkola T, Herukka SK, Blennow K, Zetterberg H, Leinonen V, Gobom J. Novel cerebrospinal fluid biomarkers correlating with shunt responsiveness in patients with idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2023 Jun 5;20(1):40. PubMed.
- Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Någren K, Tapiola T, Pirttilä T, Rinne J, Jääskeläinen JE, Soininen H, Rinne JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008 Oct;65(10):1304-9. PubMed.
- Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, Vainikka S, Pyykkö OT, Mölsä J, Fraunberg M, Pirttilä T, Jääskeläinen JE, Soininen H, Rinne J, Alafuzoff I. Amyloid and tau proteins in cortical brain biopsy and Alzheimer's disease. Ann Neurol. 2010 Oct;68(4):446-53. PubMed.
- Vera Quesada CL, Rao SB, Torp R, Eide PK. Immunohistochemical visualization of lymphatic vessels in human dura mater: methodological perspectives. Fluids Barriers CNS. 2023 Mar 28;20(1):23. PubMed.
- Huang W, Bartosch AM, Xiao H, Maji S, Youth EH, Flowers X, Leskinen S, Tomljanovic Z, Iodice G, Boyett D, Spinazzi E, Menon V, McGovern RA, McKhann GM, Teich AF. An immune response characterizes early Alzheimer's disease pathology and subjective cognitive impairment in hydrocephalus biopsies. Nat Commun. 2021 Sep 27;12(1):5659. PubMed.
- Wong DF, Moghekar AR, Rigamonti D, Brašić JR, Rousset O, Willis W, Buckley C, Smith A, Gok B, Sherwin P, Grachev ID. An in vivo evaluation of cerebral cortical amyloid with [18F]flutemetamol using positron emission tomography compared with parietal biopsy samples in living normal pressure hydrocephalus patients. Mol Imaging Biol. 2013 Apr;15(2):230-7. PubMed.
External Citations
Further Reading
No Available Further Reading
A Day's Work: Cortex Biopsy Comes Out. Shunt Goes In. Patient Goes Home.
It's 7:30 a.m. and Kuopio University Hospital is humming. On the second floor, neurosurgeons are easing into their day with a team discussion of ventriculo-peritoneal shunting, while next door, a woman in her 70s is being readied to go under the knife. In the weeks before, she entered a diagnosis-cum-research protocol. It showed she was a candidate for this therapeutic procedure while also documenting her cognition, imaging her brain in various ways, and banking blood and CSF for longitudinal research (see Part 1 of this series).

Where the Shunt Will Go. In the operating room, the patient's MRI is displayed with distance markings. [All images by Gabrielle Strobel.]
The woman is lying on the operating table, unconscious under general anesthesia. A monitor mounted above her left displays her MRI, marked up with measurements to guide her surgeon's work. The scan shows what brought her here: gaping fluid-filled holes where there should be gray and white matter. A grossly enlarged ventricle is smushing her brain. This excessive fluid pressure is what the team—a surgeon, an anesthesiologist, three nurses—is here to fix. Along the way, they will collect samples for science done in Kuopio and internationally.
The surgeon, Paula Walle, draws three incision sites on the woman's skin: one at the skull's Kocher point, behind her hair line, an inch from midline toward her right ear; another behind her ear, and a third on the right side of her belly. Walle shaves the right half of the woman's head and disinfects her skin from the waistline up. Some bacteria can hide at the bottom of hair follicles, beyond the reach of alcohol, hence the surgeon gently turns the woman's head onto her left cheek, and the nurses cover her entire upper body, save for the incision sites, with yellow sheeting that is glued to her skin. She now looks a modern-day mummy with her left hand peeking out.
Meanwhile, Ville Leinonen, the neurosurgeon who leads the hospital's hydrocephalus shunt biopsy program, checks that supplies are at hand for sample collection: tubes filled with artificial CSF for the cortical pyramid to float in on its imminent journey from the patient's brain to the sectioning vibratome (see Part 1 of this series), a Dewar filled with liquid nitrogen to freeze two needle biopsies, ice to cool the ventricular CSF, saline to rinse, and various tubes to hold all the samples that are soon to come.

All Covered Up. Preparing the patient, a surgical nurse bends a light over her abdomen.
Today, like every week, these logistics were the work of the hydrocephalus shunt biopsy program's research nurse. Tiina Laaksonen not only manages the frozen brain samples, she is also the patients' main point of contact. They know her best. Before and after their operation, she, with a neurologist, tests their gait and cognition in the outpatient clinic. Laaksonen coordinates their post-op and annual follow-up visits. She maintains the patient registry. As in most clinical research programs that thrive over time, a devoted research nurse stands at its center. "Tiina handles everything. I could not do a thing without her," Leinonen says.
At the operating table, Walle injects lidocaine-adrenaline at the incision sites. Adrenaline reduces bleeding by constricting blood vessels, especially on the highly vascularized skull. It also adds a second local pain-killing effect to the anesthesia. "If the patient felt any pain while being under, her blood pressure might go up. This injection stabilizes the anesthesia," she explains.
Walle cuts. First, an inch-wide opening behind the ear. This is where a little valve will go, to adjust the outflow of ventricular fluid. Second, an incision through the belly into the abdominal cavity, where the shunt catheter will end. This cut is when the first biopsy comes out—a bit of skin. Leinonen cleans off the blood with rinses and shakes in saline, then drops the skin into cold culture medium, right in the OR. "Just like with bloody CSF, you won't get good results with blood-contaminated skin," he says.
Next out: 3 mm lumps of fat from under the skin and, if the patient has any, intraperitoneal adipose tissue as well. Also, a tiny clipping of muscle from the abdominal wall. In 2013, while conducting a metabolic study (Takalo et al., 2014), Leinonen and Mikko Hiltunen at UEF decided that banking those tissues might be a good idea, and by now the Kuopio tissue bank stores a lot of it. "We thought at the time that the right research questions will emerge," Leinonen said. A decade later, metabolomic and lipidomic ADRD research is growing rapidly, as is its need for well-phenotyped human samples.
Next, Walle grabs a metal tunneling instrument. It is about 2 feet long. With it, she bores a path, i.e., a 4 mm-wide channel, through the patient's connective tissue between skin and facia, pushing all the way from the ear through the neck, over the chest and to the abdomen. While hard to watch for the uninitiated, this is the safest part of the procedure, Leinonen explains. It's done before the skull is opened, to minimize the time the brain is exposed to air.

Through the Burr Hole. A forceps holds back skin from a hole drilled into the skull. Just having taken a dura biopsy through this hole, neurosurgeon Paula Walle now looks to the nurse to hand her a knife, so she can cut and gently lift out a frontal cortex biopsy.
Now it's time to create a half-inch-wide burr hole above Brodmann area 8. Walle uses a trepanation drill, the kind that stops automatically when it has passed the skull. "You cannot drill into soft tissue with this," Leinonen said. Through the hole, beyond surprisingly thick walls of bone, soft white tissue comes into view. The dura. From it, a little biopsy square comes out, takes a saline shower, and dives into a Dewar.
Looking beneath the dura now, the surgeon realizes that the left half of the burr hole opens onto a sulcus. This means she will work only through the hole's right half for the remainder of the procedure. Neurosurgeons avoid sulci, because these invaginations of cortex have a nest of blood vessels at their bottom, making gyri a far safer point of entry into the ventricle.
Next, the pyramid extraction. With a dura knife, Walle cuts the pia and the soft, sticky cortical layers, aiming just deep enough to catch a tiny tip of white matter at the bottom. Experience guides her here, not technology. She lifts up the piece, ever so gingerly. "That is the critical part. If you crush it, the cells won't fire," Leinonen says. As he receives the pyramid onto plastic foil and slides it into a sterile tube filled with artificial CSF, he sounds satisfied: "It looks rather nice. I hope they get it working in Tarja's lab."

Mighty Tiny. Leinonen slides a cortical biopsy (whitish blob floating above his left index finger) into artificial CSF, taking care not to squeeze the tissue.
Now the clock is ticking. Pyramid in hand, Leinonen leaves the OR and hurries to a hospital side entrance. There, he passes it on to a junior scientist, who couriers the precious bit to Tarja Malm's laboratory at the A.I. Virtanen Institute for Molecular Sciences a half-mile away. By bicycle. (That day, Malm had brought her daughter's bike to lend to the postdoc because his was broken.)
Why the bike? "We wanted to use a drone, but they are verboten here so as not to interfere with the emergency helicopter pad on the hospital roof. So, we chose the next-fastest way," Leinonen said. (To learn what happens to the pyramid, see Part 3).
Back in the OR, Walle pulls two more pieces of cortex from the same opening into a biopsy syringe and gently expresses them into saline for rinsing. They look like tiny white worms. Within a minute, they go into liquid nitrogen. They are for snRNA-Seq analyses.
Next, Walle slides in a piece of soft silicon tubing that is as wide as the cortical pyramid she has just removed. This tube, essentially, constitutes the shunt. She knows the tube has penetrated the ventricle when CSF comes out the other end. She catches about 20 ml of it in three separate vials, which go on ice.
The sequence of which tissue the surgeon biopsies at which point in the shunt placement procedure is designed to add neither time nor risk for the patient; in other words, samples are taken during each respective step of the surgery procedure. This is standardized.
Next stop for Leinonen: the hospital lab. There he drops off vials of the patient's blood for routine diagnostic tests of protein, cell counts, glucose.
Then he heads downstairs to the tissue bank in the same building. "It's a bit of a secret place," he says. This newly constructed space houses one of Finland's five regional biobanks. The country in 2012 passed a biobanking law, which regulates how samples from outpatient medical care, research studies, and associated registries across the country are to be stored, shared, and the identities of their donors protected. Even as professor of neurosurgery at Kuopio University Hospital and head of its NPH and Early AD program, Leinonen's access is restricted. He has no key, and has never seen the liquid nitrogen tanks there.

No Key to the Bank. Leinonen waits to get buzzed into the biobank, to drop off CSF collected minutes earlier during an NPH shunt operation.
To drop off samples from the still-ongoing surgery, Leinonen gets buzzed in and hands off cortex and ventricular CSF to a dedicated neuroscience technician there. Ulla Lehtoaho will centrifuge the ventricular CSF to pellet, cryoprotect, and freeze its cells for research, then she will aliquot and store the supernatant.
Lehtoaho is busy on this Friday. In addition to samples from this morning's iNPH surgery and a second one to be done in the afternoon, she is processing CSF taken at the hospital's neurology outpatient clinic—again for dual diagnostic and research purposes—from people with Alzheimer's, FTD, Parkinson's, and related diseases.
Also rolling into the biobank today: controls. There are never enough controls in CSF research studies. Once again, to aid diagnosis of the patient at hand, and also to build a collection of comparison samples, KUH physicians draw 1 to 3 ml of CSF whenever they need to place an intrathecal catheter anyway to achieve spinal anesthesia. This could be for hip or knee replacements, gynecology, or urology procedures.
"It does not matter what is the indication. All over the world this is the best way of getting control CSF," Leinonen said. Older patients take memory tests to support their phenotypic classification as controls; many have their data in FinnGen to add genotype information to their file.

Modern-Day Bank Teller. In Finnish biobanks, CSF from NPH and many other types of surgery get aliquoted (seen here), counted and deposited for diagnostic and research purposes.
Led by Kaj Blennow and Henrik Zetterberg, scientists at UGothenberg's Sahlgrenska University Hospital have been collecting CSF in the course of routine neurology care for many years. Now, Kuopio is doing the same thing as part of Finland's broader effort building a CSF biobank. The cell-preservation protocol, an adaptation of commercial “Mr. Frosty” kits, is but the latest addition to this project.
The Kuopio group send aliquots of CSF to Gothenberg so their collaborators there can analyze larger groups for more power. The CSF cells are going to Beth Stevens and Sam Marsh at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, for research. Leinonen and Hiltunen hope that FinnGen will add CSF collection in its next project phase, too (see Part 5).

Safety in Numbers. The OR's antiseptic protocol requires fresh double gloves before catheter placement.
Back in the OR, biopsy collection is finished. Nothing more will come out of the patient. Now it's time for the valve and catheter to go in. This is the moment when, to further enhance sterility during the procedure, the surgeon removes the top set of her two gowns and gloves; a nurse helps her put on fresh pairs.
Walle lifts the shunt catheter out of the antibiotic solution in which it has been waiting. She threads this piece of tubing under the woman's skin through the previously formed tunnel from her ear to its outlet in the belly. She connects its proximal end to the lower outlet of the valve by the ear. She connects the much shorter piece of tubing she previously placed in the patient's ventricle to the valve's upper outlet, running this tubing under the skin to protect it from impact.

It's Producing! Holding the shunt valve by the patient's ear, the surgeon makes sure the distal end of the catheter (in her right hand) produces fluid before settling it into the abdominal cavity.
Walle tests that the shunt is working. "See, it is producing fluid. The ventricular end is properly in place and there is no blockage along the way," she says, holding up the tube's distal end. Then her hand reaches into the patient's abdominal cavity to make sure the catheter can move freely among the bowels. The droplets of brain fluid exiting here get resorbed amid the general moisture of the peritoneal cavity.
While closing the incisions, the surgeon calmly notes that today's all-female OR team has become “quite normal” in Finland. The country supports work-life balance like few others. Walle went on paid leave for a year when her daughter was born. Upon returning to work, she took the four-day-a-week option, to be mom on Mondays and brain surgeon Tuesdays through Fridays. Her daughter's day care cooks her a warm lunch. And it is free.—Gabrielle Strobel
References
News Citations
- Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
- Brain Biopsies and FinnGen Form Wellspring for Functional Genomics
Paper Citations
- Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, Mäkinen P, Leinonen V, Pihlajamäki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. J Nutr Biochem. 2014 Jun;25(6):634-41. Epub 2014 Mar 12 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
What little scientists know about how human neurons function in vivo comes from resections of brain tissue in people with cancer or intractable epilepsy. Scientists slice these biopsies, keep the slices viable for the day, and characterize the morphology and firing patterns of neurons in real time. In Alzheimer's research, brain biopsies became a coveted resource once it became clear that the computational capacity of human neurons is vastly different than that of rodent neurons. Demand for human tissue for research purposes rose even further when findings in AD rodent models failed to replicate in clinical trials.
Alas, human brain tissue is extremely scarce. And even when scientists can get their hands on the occasional epilepsy or tumor biopsy, neither are ideal for AD research. Epilepsy patients tend to be young adults, and both seizures and tumors confound brain structure, function, and gene expression. The medical needs of the patient at hand naturally dictate what region is resected, hence each biopsy is different, and scientists cannot build standardized sample collections needed to establish group effects.
Enter idiopathic normal-pressure hydrocephalus (iNPH). This age-related illness has become a steady source of tissue from one cortical gyrus for electrophysiologists to probe—at least for the electrophysiologists of the city of Kuopio in eastern Finland. There, research-minded surgeons, working hand-in-glove with Alzheimer's investigators, have devised a system of interlocking research protocols that enable multilevel analyses of a range of different tissues from one and the same deeply phenotyped person. Conveniently for Alzheimerologists, 40 percent of these iNPH patients—being, as they are, in their 60s to 80s—have amyloid; 10 percent have both amyloid and tau pathology. They constitute a natural model of preclinical Alzheimer's disease, with controls. Some carry causative mutations for Alzheimer's or related disorders (see Part 1 of this series).
Every Friday at Kuopio University Hospital, as patients undergo a day surgery called ventriculo-peritoneal shunt placement, the operating surgeon makes four tiny cuts into the spot on the patient's right frontal cortex where the shunt catheter needs to penetrate, gently sliding a pyramid-shaped biopsy into a Falcon tube full of artificial CSF (see Part 2 of this series). Within the hour, the cortical pyramid finds itself getting sliced and prodded with electrodes.

Layers and Cells. Nissl staining of right frontal cortex slices (upper left) shows that the biopsy includes all cortical layers, from the pia on top through layers 1 to 5/6 and white matter on the bottom. Enlarged sections show neurons in all layers. [Courtesy of Dougalis et al.]
Will its neurons be active? After all, at a diminutive 10 to 20 cubic millimeters, iNPH biopsies measure but a few percent of the typical volume of tumor or epilepsy resections, which are up to 1 cubic centimeter large. Think diamond versus sugar cube. Over the past two years, Antonio Dougalis in Tarja Malm’s lab at the University of Eastern Finland has led efforts to work out a protocol to get these cells to fire.
Now, they are active in a dish. The patients' neurons fire, both spontaneously and when provoked by drugs. They fire bursts both as individual cells, and jointly in oscillation patterns that require coherent synaptic transmission. Application of GABA reduces NMDA-induced activity. These and other basic properties resemble what previous recordings of epilepsy and tumor slices have shown, Malm said.
No Partying on Fridays
Feasibility having been established and technical kinks straightened out, the Kuopio neurophysiology team is now in data-collection mode. Every Friday, they receive one or two pyramids depending on how many iNPH patients came to surgery that day. They record away until the slices give out late at night. As they work, they are blinded to the phenotypic and genotypic information that is being gathered on the donors as part of a longitudinal research program. Without knowing if the slice before them has amyloid or tau pathology, they execute standardized recording scripts Dougalis has developed. They are piling up data in hopes of getting beyond low-N observations and toward robust group effects with statistical power.

Easy Does It. Anssi Pelkonen sets the vibratome blade to approach a human brain sample. He chooses a slow 0.10 mm/s, to avoid cellular damage so the neurons inside stay fit to fire. Right, blade shaves slice off a tiny 350 micron-thick slice. [Courtesy of G. Strobel, A. Dougalis.]
In a year or so, when the different types of data being gathered on a given person can be integrated, the scientists are hoping for big insights. "At 50 biopsies per year in Kuopio, to gather sufficient N to see group differences takes time," Malm said. Because the small size of the biopsies precludes extensive tissue sharing for acute-slice neural activity studies, progress would accelerate if other hospital-associated research centers set up similar systems, she added.
That said, Dougalis and Malm have already gleaned early insights from their analysis of the first 55 biopsies over the past year, some with AD pathology, some without. Tantalizingly, it appears that findings at the functional level jibe with findings at the RNA sequence level in the same person.
When fresh brain arrives from the operating room in the Malm lab, postdoc Anssi Pelkonen promptly embeds it in agarose, mounts it in a vibratome, and slowly moves the blade across the block, shaving off 350-micron-thick slices. He sorts them for different uses. The first, irregular bits are frozen for proteomics. One slice gets fixed and returned to the hospital pathologist to support the patient's diagnosis. One slice is for research immunohistochemistry in the Malm lab, one is for EM, one for spatial transcriptomics.

Top: Mireia Gómez-Budia explains that the mesh shown on her left screen holds down the cortical slice, to make sure the electrode tips sticking up from below are penetrating. Bottom left: Slice of human cortex layers 1 to 6 mounted on three-dimensional multi-electrode array under microscope. Bottom right: MEA channel map, enlarged below a titanium nitride electrode tip. [Courtesy of G. Strobel, A. Dougalis.]
Two adjacent slices are used immediately, one for single-neuron patch-clamp recordings to characterize the behavior of individual neurons, the other for multi-electrode recordings to characterize neural circuits and their higher-order functions. While Pelkonen sorts the slices, their donor in the hospital is about to be wheeled from the OR to the recovery room.
Ph.D. student Mireia Gómez-Budia straps one slice onto a multi-electrode array (MEA). Its 60 electrodes poke into neurons from below, across the entire depth of cortex. Once mounted, the slice accommodates to its new surroundings in oxygenated saline for a half hour before Gómez-Budia puts it to work. She checks activity parameters from action potential burst rates to local field potentials. She records multi-unit activity, i.e., the summed activity of all neurons near a given electrode or multiple electrodes, respectively. These measures characterize more complex circuit functions such as gamma or theta waves.
Some slices fire spontaneously, Gómez-Budia says. Others get active after she stimulates them with NMDA to awaken glutamatergic synapses, or with carbachol to stimulate cholinergic ones.

My Neuron and I. Polina Abushik spends her Fridays in a dark electrophysiology cave, poking a living woman's pyramidal neuron with a patch-clamp electrode, and recording what it has to say.
Searching for a memory mechanism of sorts, Gómez-Budia tries to induce LTP in the slices. Via the MEA, she stimulates cortical layers 4 or 5 and records in layers 2/3. "We have data of slices that are potentiating and inducing LTP," she told Alzforum.
Five meters away, postdoc Polina Abushik tries to get a single electrode to form a seal with the cell membrane of a layer 2/3 pyramidal neuron for her patch-clamping session with the cortical slice adjacent to Gómez-Budia's. She hopes her electrode will penetrate an individual neuron so she can record the goings-on, ranging from its resting membrane potential, action potential threshold, frequency, and other electrophysiological properties.
"You can hear how one neuron works," Abushik says. First she listens in on the cell's intrinsic activity, then she documents evoked responses to current she injects via a stimulating electrode placed on the neuron's “cheek,” aka the side of its soma. To build homogeneous data sets that allow conclusions about specific cell types, the scientists started in layers 2/3, patching dozens of pyramidal and interneurons. The latter are quite different morphologically and functionally, and tricky to record, Malm said.
Snce a slice is “exhausted” and the recording session ends, hopefully after many hours of data collection, the scientists don't toss it. It's still precious tissue. They fix MEA slices for histology, to relate the presence of, for example, amyloid or tau pathology, to the neurons' documented activity. They fill patched neurons with the neuron stain biocytin and then visualize their arborization and dendritic spines. Already, the group has documented a far greater variety of morphological differentiation than previously seen in mouse cortex.

Shapes and Sizes. Post-recording morphometric reconstructions of cortical neurons from living shunt recipients. Cortical layers, and cortical depth in millimeters, are indicated on left. Pyramidal neurons are shown with their apical dendrites in blue and basal dendrites in red. On the right, two interneurons. [Courtesy of Dougalis et al.]
Overall, Dougalis' feasibility study found that just under half the iNPH biopsies yield slices that appear to have normal intrinsic, synaptic, morphometric, and network properties. They exhibit intact excitatory and inhibitory synaptic transmission, but no abnormal epileptiform hyperexcitation. The circuitry to support high-power theta- and gamma-band oscillations is preserved. Curiously, across the age range available in this collection of samples, the capacity of cholinergic transmission to induce gamma-band activity was weaker in older than in younger people. In toto, the scientists conclude, this protocol is suitable for detailed functional exploration of adult human neurons.

How They Fire. Simplified properties of example interneuron (top) and pyramidal neuron (bottom) from a living older person's frontal cortex. The interneuron fired faster and narrower action potentials than the pyramidal neuron (column B). In voltage clamp, the interneuron's electrophysiological trace sustained a higher frequency (column C). Both neurons had spontaneous synaptic activity, and synaptic depression after repetitive stimulation (column D).
Regarding those deeper explorations, analyses are at early stages, but Malm noted some budding trends. One comes from her collaborators Evan Macosko and Beth Stevens at the Broad Institute, Cambridge, Massachusetts. They receive small bits of tissue from the same cortical location from the same Kuopio shunt recipients for transcriptomics analyses. When sorting the biopsy's cell types based on expression signatures, the Broad group noticed that a particular population of interneurons was mysteriously absent from cortical layer 1 in people who have amyloid pathology there.
To learn more about this, Malm’s team stimulates layer 1 in the biopsies. They want to know if there is inhibitory input from layer 1 into deeper layers such as 2/3, and whether that might weaken in people who have amyloid pathology nearby. In theory, if a set of interneurons, which are usually inhibitory, is missing, then their target neurons can become disinhibited and hyperexcitable. "Based on the RNA-Seq finding that a type of L1 interneuron is lost in brains with Aβ burden, we would expect to see a difference in L1 operational characteristics, and in L2/3 drug-induced responses," Malm said.
Importantly, Dougalis is already starting to see changes in markers of connectivity in people with Aβ pathology. By this he means connectivity within a slice, not the brain-wide connectivity as measured commonly by fMRI. Dougalis deduces connectivity based on analyses of spike cross-correlation and local field potential coherence probability. These analyses enable a comparative interpretation of the signals coming from each of the MEA's electrodes. When Dougalis adds NMDA or carbachol, he sees drug-specific and band frequency-specific changes in coherence probability and a reduction in the electrodes with cross-correlated spike trains. Both were related to AD pathology. "This indicates some degree of loss of connectivity in amyloid-positive brains," Malm said.
This increased excitability at the transcriptional level of layer 2/3 pyramidal cells with Aβ pathology seen Macosko’s lab is echoed by the data in Malm's lab. Specifically, the loss of L1 interneurons led to changes in the oscillatory local field potentials in that layer, and to a hyperexcitable phenotype in layer 2/3 based on spiking activity.
This implies that in the superficial layers of the cortex of a given person, Macosko and Malm see the same thing, he with RNA, she with physiology. How the added presence of tau pathology may affect all this is a focus of intense curiosity in the lab these days; thus far, Dougalis and colleagues have recorded from about a dozen such patients.
To see how layer 1 interneurons and layer 2/3 excitatory neurons feature in Macosko and Steven's first big study of these biopsies, see Part 4.—Gabrielle Strobel
Comments
No Available Comments
Make a Comment
References
News Citations
Further Reading
Cortical Biopsies Hint at Start of Alzheimer's 'Cellular Phase'
What goes on inside the brain of a person in the preclinical stages of Alzheimer’s disease? Science grapples with this question by studying postmortem tissue, despite concerns about agonal changes and postmortem interval degrading sample quality. Now, a unique collection of surgical biopsies offers a fresh perspective. In a manuscript uploaded June 5 to bioRxiv, researchers led by Evan Macosko and Beth Stevens at the Broad Institute of MIT and Harvard report the first single-nucleus RNA-Seq analysis of cortical samples that were were taken during surgery to implant a ventricular shunt to relieve symptoms of hydrocephalus.
Integrating this new biopsy data with prior postmortem transcriptomic data, the scientists compiled an enormous set of cell profiles spanning disease states and species. From this emerged a cellular signal of early AD, in living people, that has never been seen before.
It reveals loss of a particular set of layer 1 inhibitory neurons along with transient hyperactivation of a particular set of layer 2/3 excitatory neurons. Amyloidogenic APP processing ramps up in pyramidal neurons and oligodendrocytes, and a specific cluster of microglia expands. The dataset offers a glimpse into the cellular phase of AD, a years-long debacle that unfolds after amyloid forms and during which malfunctioning neurons, glia, and endothelial cells damage the brain (De Strooper and Karran, 2016).
“What sets this data apart is that we found some credible biological insight into early Alzheimer’s,” Macosko told Alzforum. “We have seen clear evidence of the hyperactivity hypothesis in AD brain.”
“This exciting study [...] stands out methodologically,” noted Martin Kampmann, University of California, San Francisco. Rick Livesey, University College London, cautioned that the donors' normal-pressure hydrocephalus may complicate interpretation of the results, but called the overall work "a welcome and insightful contribution to understanding the cell and molecular biology of dementia in humans in vivo."
Like many a good collaboration, this study was born at a conference, Macosko told Alzforum. Wanting to run large-scale transcriptomics, but concerned about the limitations of postmortem tissue, Macosko wished for a cohort of good ex vivo samples. Henrik Zetterberg, from the University of Gothenberg, Sweden, knew just whom to ask. He introduced Macosko and Stevens to Ville Leinonen, a neurosurgeon at the University of Eastern Finland, in Kuopio. For years, Leinonen has been collecting, storing, and characterizing samples of frontal cortex taken during hydrocephalus shunt placements (see Parts 1 and 2 of this series).
“We have worked with other surgical samples before, but Ville’s attention to detail is unparalleled, and he has a personal interest in obtaining the highest quality data,” said Macosko.
With tissues biopsies from 52 donors, frozen within five minutes of coming out of the brain, joint first authors Vahid Gazestani and Tushar Kamath and colleagues used single-nucleus RNA-Seq to profile the transcriptomes of nearly 900,000 cells, or 17,000 per donor. Nineteen samples had amyloid plaques, eight had plaques and evidence of phosphorylated tau, 25 had neither. By clustering like transcriptomes, the scientists identified the major cell types: excitatory and inhibitory neurons, microglia, astrocytes, endothelial cells/pericytes, oligodendrocytes, and oligodendrocyte precursors. Further clustering within each produced 82 subtypes.
The scientists reasoned that with this high-quality dataset, they'd have enough power to integrate and characterize previous data with greater specificity. From 27 published studies, almost 2.5 million cells met quality-control criteria and were annotated based on the 82 cell subtypes identified in the Kuopio cohort. The prior analyses were of human postmortem samples of people with AD, Parkinson’s, multiple sclerosis, and autism, plus datasets from mouse models of AD and related disorders.
What did this analysis reveal? First, the comparison of fresh to postmortem tissue confirmed that the morbidity just before death, and degradation after, had indeed changed gene-expression patterns in the prior datasets. For example, it rendered inhibitory and excitatory neurons less complex, and generated expression artefacts in microglia.
How about an AD signature? By correlating gene expression with pathology burden, Gazestani and colleagues recognized cellular changes specific to preclinical AD. First, they noticed that two specific types of neuron were depleted in cortex with amyloid but not tau pathology. They are interneurons expressing the genes NDNF and PROX1, and excitatory neurons expressing LINC00507 and COL5A2. These neurons lie in layer 1 and layers 2/3 of the cortex, respectively.

Early Losses. In frontal cortices from living people who have only amyloid pathology, two groups of neurons are depleted: those expressing NDNF-PROX1, and those expressing LINC00507-COL5A2 (left). In people who have both amyloid and tau pathology, there are more microglia expressing GPNMB and LPL genes, and fewer microglia expressing CX3CR1. [Courtesy of Gazestani et al., bioRXiv, 2023.]
In people with high amyloid pathology plus tau phosphorylation, the relative dearth of these neurons was no longer evident. Macosko suspects this is because by this later stage of pathology, other neurons start dying, too.
Are these cellular changes coordinated? A look at gene-expression patterns in the excitatory neurons suggests as much. The authors found two pools of differentially expressed genes, aka reactomes, in these cells. One emerged in cortices that had only amyloid pathology, and petered out in cortices that had both amyloid and tau pathology. The second surfaced once both pathologies were present.
Gene set enrichment analysis identified pathways behind these reactomes. When amyloid burden was present, the layer 2/3 LINC00507-COL5A2 excitatory neurons ramped up genes needed for glucose metabolism, the tricarboxylic acid cycle, and mitochondrial electron transport—as if they were trying to meet demand for more energy. When amyloid burden was high and phosphorylated tau had entered the picture, expression of these same genes waned, suggesting the neurons were struggling to survive. Cortical neurons in deeper layers mounted much weaker gene-expression changes, another indication that this particular neuronal response might be quite specific (image below).
Layer 2/3 LINC00507-COL5A2 excitatory neurons also transiently activated cell protective genes, e.g., those for cholesterol synthesis, scavenging reactive oxygen species, DNA repair. Tellingly, loss of the L1 NDNF-PROX1 inhibitory neurons correlated with these expression changes. In addition, the fewer inhibitory neurons were in the sample, the more the excitatory neurons ramped up genes typically induced after neural activity, such as FOS and JUN. This hints that the loss of this L1 inhibition increases activity in L2/3 excitatory neurons.

Spot the Reactome. Columns show types of excitatory neurons, each with three levels of increasing AD pathology from left to right. Upper layer LINC00507-COL5A2 and RORB_SCTR neurons respond (Y axis) to amyloid and tau pathology, but only transiently. Expression of genes needed to meet energy demands (rows, see glucose metabolism, TCA cycle, electron transport) turns up (red dots) when amyloid pathology (x axis) is present, but peters out (blue dots) when amyloid burden is high and tau is phosphorylated. Reactomes protecting against reactive oxygen species and DNA damage are also transiently activated. Lower layer neurons do not react this way. [Courtesy of Gazestani et al., bioRXiv, 2023.]
“Collectively, our results demonstrate NDNF-PROX1 inhibitory neuron loss is correlated with hyperactivity and preferential loss of layer 2/3 excitatory neurons in the prefrontal cortex with low Aꞵ plaque burden,” the authors concluded. Scientists have long known of paradoxical hyperactivation in early AD and animal models, and evidence has implicated loss of inhibitory input (Palop et al., 2007; Busche et al., 2008; Putcha et al., 2011; Verret et al., 2012).
Gazestani et al. call their finding the Early Cortical Amyloid Response. It has additional components. Take the 400,000 microglia in this study. Their transcriptomes fell into 13 subtypes, of which two—marked by LPL/CD83 and GPNMB/EYA2 expression—were becoming relatively more numerous with increasing pathology. The latter subtype also behaves this way in PD, and in mouse models of ADRD, ALS, and multiple sclerosis, suggesting these microglia represent a general response to pathology or neurodegeneration. Expansion of LPL/CD83 microglia appears specific to AD.
Another change jumped out. When there were plaques, genes responsible for Aβ production, including APP itself, were turned up. The authors were surprised to see this not only in the hyperactive excitatory neurons, but also in oligodendrocytes. For both cell types, the signature was strongest in samples with the lowest amyloid burden, suggesting a response to early pathology. Genes known to temper Aβ production, including SORL1 and PICALM, were down (image below).

Trouble Brewing. According to analysis of a set of 45 genes involved the production of Aβ, hyperactive neurons and oligodendrocytes in living people with AD pathology make more of the peptide. [Courtesy of Gazestani et al., bioRXiv, 2023.]
Macosko thinks perhaps the oligodendrocytes ramp up Aβ production because they are trying to assist the neurons. “APP production as a fraction of total expression is higher in oligodendrocytes than in neurons, so the glia are putting a lot of energy into making it,” noted Macosko. “I think the uptick is probably non-autonomous. It may be a response to the hyperactive neurons, but we don’t know that yet."
Are these early changes a response to amyloid burden? That’s the assumption, but Macosko said it is still a mystery. Unclear also: whether the loss of inhibitory neurons precedes, or causes, the hyperactivation and loss of excitatory neurons. “Right now, all we have is a correlation,” he said.
Neither does he know why these particular neurons are affected. The scientists searched for shared characteristics that might explain their vulnerability, such as ion channels or patterns of activity or expression. Nothing stood out. Perhaps these neurons' position in the outer layers, closer to the brain border, may expose them more to cytokines and other molecules that might pose a threat. Small samples of dura are also being collected during shunt surgery, and starting to be studied.
Or perhaps the neuron's circuitry makes them vulnerable to stress because they receive inputs from relatively far away. Further studies might tell. “We now have a system where we can probe these cells electrophysiologically in acute slices to ascertain their excitability and transcriptional response. That opens the door to exciting opportunities,” he said (see Part 3 of this series).
Meanwhile, the biopsy donors live on, as participants in a longitudinal NPH research program.—Tom Fagan and Gabrielle Strobel
Comments
No Available Comments
Make a Comment
References
News Citations
- Fresh Brain Every Friday: Biopsies Transform Alzheimer's Science
- A Day's Work: Cortex Biopsy Comes Out. Shunt Goes In. Patient Goes Home.
- Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
Paper Citations
- De Strooper B, Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2016 Feb 11;164(4):603-15. PubMed.
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007 Sep 6;55(5):697-711. PubMed.
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008 Sep 19;321(5896):1686-9. PubMed.
- Putcha D, Brickhouse M, O'keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J Neurosci. 2011 Nov 30;31(48):17680-8. PubMed.
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012 Apr 27;149(3):708-21. PubMed.
Further Reading
No Available Further Reading
Brain Biopsies and FinnGen Form Wellspring for Functional Genomics
Tucked into the waterways of a Nordic landscape, moose roaming its woods near the Russian border, Kuopio, population 300,000, is both small and remote. Yet this Finnish city lies in the center of a new approach to functional genomics and personalized medicine research on the global scourge of age-related neurodegeneration. Its secret? Close integration between neurosurgeons and all manner of biologists, plus data collected across national health registries, tissue banks, and genotyping cohorts.
In this technologically advanced nation of 5.5 million people, Mikko Hiltunen directs the University of Eastern Finland's overarching Neuroscience Research Community, UEF NEURO-RC. Among its principal investigators, a select handful collaborate on a range of functional studies that draw on Kuopio's unique practice of extensive biopsy collection and tissue banking. These, in turn, draw on routine brain surgeries that treat age-related idiopathic hydrocephalus (iNPH). UEF NEURO-RC's system of interlocking biopsy and basic research enables multilevel exploration of research questions across tissue types and through multiple levels of analysis, from electrophysiology of brain slices to transcriptomic, proteomic, and biomarker research of brain parenchyma and fluid samples. (For more on this system, see Parts 1, 2, 3, and 4 of this series.)
ADRD genetics boasts a growing list of GWAS hits. The painstaking work of figuring out how each of these operate in cells challenges scientists at UEFs Neuro-RC and around the world. Hiltunen's group has coupled Kuopio's hydrocephalus biopsy banking protocol and its attendant phenotypic data to a translational and mouse research program he calls Personalized Medicine for Microglial-associated genetic variants in Alzheimer's Disease. Aka PMG-AD, the program stands on three legs: the iNPH sampling protocol, FinnGen/Finnish biobanks, and mouse models. PMG-AD was established in collaboration with the German Center for Neurodegenerative Diseases, University of Lille, France, University of Helsinki, and Turku PET Centre, Finland.

PMG-AD. This research program taps cohort studies for carriers of genetic variants of interest, and invites them into longitudinal imaging studies that use their blood for cell-based and multiomic analyses (top). Mouse lines of those variants add a model for deeper mechanistic studies (bottom). [Courtesy of M. Hiltunen.]
At its core, PMG-AD takes discoveries from human genetics, for example the protective A673T "Icelandic" APP or PLCG2 Pro522Arg variants, finds carriers among Finnish population and biobank cohorts, and asks them to join detailed longitudinal aging studies. Likewise, carriers of AD and FTD risk variants, e.g. ABI3Ser209Phe or GRN rs5848, get invited to come to UEF or the University of Helsinki for research monitoring their prodromal stages. Ditto for carriers of the C9ORF72 hexanucleotide expansion, or TREM2 or TYROBP mutations.
Hiltunen's long-term goal is to gather rare variant carriers within Finnish biobanks into a cohort study that characterizes their preclinical pathophysiology with all major tools available, probing biomolecules, cells, tissues, and the brain. The idea draws inspiration from what the Dominantly Inherited Alzheimer's Network (DIAN) has pioneered for autosomal-dominant AD. Fortuitously for Hiltunen's team, carriers of all the above gene variants showed up not only in Finnish population cohorts but also in the hydrocephalus cohort led by neurosurgeon Ville Leinonen of Kuopio University Hospital. This means their shunt biopsies supply brain tissue and fluids for PMG-AD research projects (see Parts 1 and 2).
Can researchers pull this off in real life? It appears so, according to a pilot FinnGen recall study, albeit not yet with preclinical variant carriers, but with people whose records suggest they have early AD (Julkunen et al., 2023). Also helpful here is Finland's 2012 Biobank Law, which enables participants to donate samples with a broad consent to medical research.
A “younger sibling” to the better-known U.K. Biobank, FinnGen started in 2017. This pre-competitive partnership unites nine Finnish biobanks and five Finnish university hospitals into a hub co-funded by the Finnish government and 13 international pharma companies. Led by Aarno Palotie of the University of Helsinki and Massachusetts General Hospital, the project's stated goal is to include 500,000 Finns, nearly 10 percent of the country's population, by the end of 2023. For phenotype data, FinnGen takes digital records from hospital visits, drug purchases/reimbursement, and from longitudinal health register data collected for every resident of this Nordic nation since 1969. For genotype data, FinnGen uses legacy or newly collected blood samples and runs them on a Thermo Fisher custom array tweaked toward the Finnish gene pool.

Banks Everywhere. Nine Finnish Biobanks, seven regional ones, and three nationwide ones (gray circles) supply FinnGen with samples. [Courtesy of Nature.]
Genetically speaking, FinnGen's population is more homogeneous than the U.K.'s, because Finland lacks the population admixture arising from the U.K.’s colonial history. This makes rare, high-impact disease variants easier to find in the Finnish population.
This is not the only difference. Starting in 2006, the U.K. Biobank sought participants by mail and enrolled them with structured, in-person interviews. Hence it has detailed, standardized phenotypic and behavioral data gathered at those visits, but suffers from "healthy volunteer bias." In contrast, FinnGen pulls samples from the country's extensive network of biobanks and taps digital records from hospital visits and research studies. This makes FinnGen stacked with people who have seen specialists or been in hospitals (Rodosthenous et al., 2022). For example, a published analysis of the first 224,737 participants, median age 73, put FinnGen's Alzheimer's disease prevalence at 2.7 percent compared to 0.2 percent in the U.K. Biobank (Kurki et al., 2023). This number is based on data release 5, which is public. Data freeze 11, accessible to member scientists, already shows three times as many AD cases, Hiltunen told Alzforum.
FinnGen works by give and take. Hiltunen contributed DNA on 2,000 AD patients, plus age-matched controls, whom his genetics group has been studying over the past 20 years. Likewise, Leinonen contributed data and samples of 1,000 hydrocephalus patients. Importantly though, Hiltunen also pulls information from FinnGen to drive his lab's PMG-AD project. FinnGen is where scientists can access data and samples from individual Finns with gene variants of interest. As Hiltunen monitors the growing FinnGen data, he frequently finds, and tests, carriers of neurodegenerative variants that are being newly discovered in other genetic studies, such as the European Alzheimer and Dementia Biobank (EADB). These are some of the people he hopes to invite to join research before their disease manifests itself—or to learn how their variant keeps them cognitively well into old age.
The third leg of this PMG-AD approach is work with mouse models. Scientists led by UEF's Mari Takalo subject mice and their tissues to the same experiments she runs on samples from biobanks and iNPH variant carriers. These days, she focuses on the topic that preoccupies ADRD scientists around the world, that is, the nexus between the innate immune system, glia, and neurons during the “cellular phase” of early AD. "Whichever variant we are studying, we are characterizing if it influences key microglial functions such as phagocytosis, inflammatory response, lipid metabolism, or viability of the microglia," Takalo told Alzforum.
Her team's methods range broadly from PET imaging to cell-based assays derived from carrier monocytes, iPSCs, or organoids, and to multiomic analyses. In cases where the stars align and both FinnGen and the iNPH cohort harbor carriers of a variant of interest, Kuopio scientists have access to an unparalleled collection of tissues: frontal cortex and dura, intraventricular and lumbar CSF, cells thereof, blood and skin-derived cells including monocytes, fibroblasts to be used to generate iPSCs and organoids. All this from a living, aging carrier who is being followed cognitively, with neuroimaging and further fluid sampling as needed for their care.
This armamentarium enables the scientists to conduct frequent cross-checks to ensure that a new finding in mice is true in humans, i.e., is relevant to disease, or that a finding in humans can be experimentally explored in greater depth in mice. Hiltunen hopes this approach will fill in the sought-after pathways that lead from genes to AD, CAA, or ALS/FTD.
To illustrate how this concept works in the lab, this story summarizes four example projects: on APP, PLCg2, progranulin, and C9ORF72. Similar work is happening with Abi33, TREM2, and TYROBP. Work is ongoing, or at early stages.
APP
First described in 2012, the APP A673T variant protects against AD (Jonsson et al., 2012). Naming it the "Icelandic mutation" was perhaps a tad hasty, as it occurs in about 30,000 lucky Finns. Six years ago, when Hiltunen and colleagues submitted a paper showing that A673T carriers had 30 percent less Aβ42 in their plasma, reviewers wanted to know if that was true in their brains and CSF, as well. "We were unable to answer that because we had no such tissue in our original genetic sample," Hiltunen recalled (Martiskainen et al., 2017).
Then three APP A673T carriers cropped up in the iNPH biopsy cohort. Lo and behold, their CSF was indeed low in Aβ42. "Typically in AD, CSF Aβ42 reduction is taken to mean amyloid deposition in the brain," Hiltunen teased. But no, their brain biopsies, taken during shunt surgery (see Part 2), showed the cortices of these aging carriers to be free of amyloid pathology. In their CSF, sAPPβ, the product of BACE cleavage, was lower, while sAPPα, the product of α-secretase cleavage, was higher than in well-matched noncarriers.

Like Blood Like Brain. sAPPβ and Aβ42 levels are lower, sAPPα is higher, in CSF samples of APP A673T variant carriers (light bars) than controls (dark bars). [Courtesy of Wittrahm et al., 2023.]
Also consider results of mass-spec-based proteomic, phospho-proteomic, and RNA analyses of CSF and frontal cortex in the iNPH biopsy cohort. Rebekka Wittrahm, Takalo, and colleagues in the lab matched the cohort's three APP A673T carriers with three noncarriers of the same APOE genotype, sex, and age. They added Braak-staged AD postmortem tissue for another comparison. They found that specific pathways that were upregulated in the APP A673T carriers were downregulated in their fellow noncarriers and even more so in postmortem AD brains of increasing disease stage. "We are seeing the same pathways changed in opposite ways in living APP Icelandic variant carriers versus pathological Alzheimer's cases," Hiltunen said.
Notably, subunits of protein phosphatases that dephosphorylate tau were at several-fold higher levels in the cortices of protective variant carriers than in AD cases, possibly tying non-amyloidogenic APP cleavage to tau modification. The variant's impact appears neuronal, as microglial phagocytic capacity and maintenance seemed unchanged in APP A673T carriers. Ditto for their astrocytes; the study has not looked at oligodendrocytes, though. (For details, see Wittrahm et al. 2023).
In cell-based experiments pitting the protective Icelandic variant against both the pathogenic Swedish and London APP variants, A673T proved “stronger.” It overpowered both these mutations by lowering sAPPβ and raising sAPPα. "Wherever you look, this mutation has beneficial effects, and we confirmed that it operates through APP cleavage," Hiltunen said.
These data would appear to support BACE inhibition as a therapeutic approach. Pharma companies have not revived work on their BACE inhibitor drugs, which scientists agree could work safely at low doses that aim for no more than 30 percent Aβ reduction—in the range Wittrahm reported to be the case in APP A673T CSF.
Following his 2017 report of Aβ42 reduction in the blood of such carriers, Hiltunen received pharma requests to measure Aβ42 in the CSF of FinnGen participants carrying APP A673T. He was unable to do so at the time because FinnGen does not collect CSF, and requesting a lumbar puncture from healthy people for research is not permitted in Finland. So, it was the iNPH cohort that opened a window into the APP A673T brain and answered the old question about its effect in CSF. As FinnGen is nearing its first goal of screening and genotyping 500,000 people, the Kuopio scientists are hoping that CSF sampling can be added in its next phase. "This will help us get endophenotype data so that we can connect participants' genetics to their phenotype," Hiltunen told Alzforum. Meanwhile, a deeper analysis of the APP A673T carriers' cognitive preservation as they age is ongoing, Hiltunen said.
PLCγ2
In the current iNPH cohort, two shunt recipients carry a rare variant that fascinates scientists because it protects against AD, FTD, and Lewy body disease. Unlike APP A673T, this one works through microglia, not neurons. It is the P522R coding variant of PLCG2, which encodes the membrane-associated phospholipase C gamma 2 (PLCγ2). In microglia, PLCγ2 acts downstream of TREM2 in that receptor's signaling pathway. Brain from these two iNPH shunt recipients show no sign of amyloid pathology, Takalo told Alzforum; their tissues are banked and awaiting deeper analyses.

Microglial Pathway. Signal transduction downstream of the microglial TREM2 receptor is drawing intense scrutiny these days. The Kuopio team is studying DAP12 and PLCγ2 variants found in FinnGen and iNPH cohorts. [Courtesy of M. Hiltunen.]
In their mechanistic exploration of the PLCγ2-P522R variant, the Kuopio scientists have previously reported that it activates microglia in mice, as per gene expression, functional assays, and TSPO PET (Takalo et al, 2020). Since then, their respect for its prowess has only grown. It turns out that among its carriers in the Finnish FINGERS cohort are several asymptomatic individuals, who are cognitively normal in their 80s despite also having two APOE4 alleles.
How could PLCγ2-P522R possibly override the effect of ApoE4? For one, the scientists are finding that the lipid droplets that form in human PLCγ2-P522R microglial cultures stressed with LPS or myelin are smaller. It appears they do not fuse with larger lipid droplets. For another, PLCγ2-P522R transgenic mice express more of certain fatty-acid-binding and cholesterol-metabolizing genes. In essence, they appear better equipped to eliminate waste products as they age.
Feeding this interest in lipid metabolism is a related PMG-AD project with iNPH/FinnGen participants. It looks at pathogenic variants in TYROBP. This gene encodes the Dap12 receptor wedged between Trem2 and PLCγ2 in the same microglial signaling pathway. Using biopsy tissues, the scientists are currently extending a prior study (Natunen et al., 2020). It reported low microglial clustering around plaques in Type 2 diabetes induced in AD mouse models, and in amyloid plaques of iNPH patients with diabetes. Dap12 and PI3K-Akt appear to be involved. PI3-Akt also showed up in Takalo's lipid droplet study of PLCγ2-P522R cells.
For mechanistic studies of how PLCγ2-P522R operates in the presence of amyloid, these mice have been crossed to APP/PS1 delta E9 transgenic mice; analyses are ongoing. Will their plaques be denser, dystrophic neurites fewer, synaptic pathology milder? Early hints are that the crosses do have less amyloid. It looks as if PLCγ2-P522R microglia more actively surveil and compact plaques, but most types of tissue are still being analyzed, Takalo said.
How can scientists study people with a rare, protective variant? It's a conundrum because, by definition, there are few of them, and they don't have the disease in question. Once again, Hiltunen is looking to FinnGen. One question he wants to ask: Might the B cells of PLCγ2-P522R be mounting a better antibody response to the presence of accumulating Aβ? After all, peripheral immune cells express PLCγ2, as well. B lymphocytes make it as part of their maturation while fighting an immune challenge. "We assume that while the B cells are more activated in these carriers, they produce a factor that is beneficial in the periphery, then enters the brain," Hiltunen said. A separate aging cohort study, led by Henne Holstege at Vrije Universiteit Amsterdam, also detected a slightly more responsive immune system, including more sensitive B cells, in PLCγ2-P522R carriers (Diks et al., 2023).
Progranulin
The GRN gene popped up as genome-wide significant in an EADB GWAS FinnGen cohort (Bellenguez et al., 2022). Its rs5848 variant increases risk of ADRD. Fourteen years ago, Hiltunen's group had reported that rs5848 brings down the age of AD onset in men—to great skepticism, as he recalls (Viswanathan et al., 2009; Kämäläinen et al., 2012).
Luckily, the GRN rs5848 variant occurs among Leinonen's iNPH shunt patients. Progranulin protein levels wane in men with age, and the rs5848 variant decreases blood levels by another 25 percent in those homozygous for the risk allele. This puts them at risk of neurodegeneration, though this protein reduction is a subtler phenotype than a loss-of-function mutation or a mouse knockout.
In brain biopsy tissue of 60- to 75-year-old men with iNPH who were homozygous for the 5848 TT risk allele, Heli Jeskanen in Hiltunen's lab detected a trend toward lower progranulin protein levels than in controls. She also noticed that the number of microglia was the same as in controls but, curiously, more of them seemed to be heading toward amyloid plaques, which appeared smaller, more rounded. Studying microglia-like cells the scientists derive from these same patients' monocytes, Jeskanen found that rs5848 carrier cells took up more myelin and got more riled up by LPS. Something about them seemed more reactive. These data replicate, in human cortex and blood cells, a progranulin knockout/APP transgenic study that had reported hyperactive microglia (Goetzl et al., 2019). "This is translation from mouse to human," Hiltunen said. More work is ongoing.
Another variant of intense interest that occurs in both FinnGen and the Kuopio iNPH cohort is TMEM106B rs13237518 (Katsumata et al., 2022). Hiltunen suspects TMEM106B and progranulin act in the same pathway, and his team is dissecting the mechanisms of both genes using the brain biopsies, CSF, and plasma samples from the iNPH patients. The cortical biopsies are being explored functionally in Tarja Malm's electrophysiology group (see Part 3); the CSF and plasma from carriers are studied in collaboration with Henrik Zetterberg and colleagues in Gothenberg, to look for changes in proteome and in markers of inflammation and synaptic damage.
"We bank these tissues, hence have access to additional material for other studies. Whatever comes up in CSF, we can check whether it also happens in cortex, with gene expression or function there, or whether it correlates with something we see in plasma," Hiltunen said.
Last But Not Least: C9ORF72
iNPH biopsies can pry open the door to identifying people with structural mutations—expansions, inversions, or deletions—that GWAS cannot pick up. Consider the C9ORF72 hexanucleotide expansion. It is the most common cause of FTD in Finland. But how can scientists find its carriers before they show symptoms? Some carriers are likely in FinnGen. Alas, FinnGen determines only SNPs. The elaborate genetic methods needed to detect the C9ORF72 expansion can't be applied to this many people. "We cannot see C9 expansions in FinnGen," Hiltunen said.
Find a Needle in the Haystack. Kuopio’s iNPH cohort enabled a genetic search strategy to pick out likely C9ORF72 hexanucleotide expansion carriers in FinnGen.
As it happened, the Kuopio iNPH cohort contains eight carriers (Korhonen et al., 2019). A genetic bootstrapping hunt ensued. Leinonen shared DNA and data from these carriers with the EADB. The world's largest AD genomic consortium, EADB continues to surface new disease variants (e.g. Nov 2022 news). Knowing which iNPH shunt patients had the C9ORF72 expansion enabled Hannah Rostalski in Annakaisa Haapasalo's lab at UEF to determine which GWAS proxy markers in EADB are associated with this expansion. Those proxies helped her define co-inherited SNP combinations, aka haplotype blocks, that can flag any person as likely carrying a C9ORF expansion. Once plugged into FinnGen, which at the time of this hunt had genotype data on 218,792 Finns, these haplotypes pulled out candidates. When the scientists checked these candidates' clinical diagnoses, lo and behold, they had FTLD and/or ALS (Rostalski et al., 2021).
Haapasalo and colleagues are now inviting these FinnGen participants, both those with a diagnosis and asymptomatic expansion carriers, into a research study to formally confirm the expansion in them with clinical-grade testing, and then to study their progression as part of UEF's translational medicine program.—Gabrielle Strobel
References
News Citations
- Fresh Brain Every Friday: Biopsies Transform Alzheimer's Science
- A Day's Work: Cortex Biopsy Comes Out. Shunt Goes In. Patient Goes Home.
- Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
- Cortical Biopsies Hint at Start of Alzheimer's 'Cellular Phase'
- Rare Variants in Lipid Transporter Genes Increase Risk for Alzheimer’s Disease
Mutations Citations
Research Models Citations
Paper Citations
- Julkunen V, Schwarz C, Kalapudas J, Hallikainen M, Piironen A-K, Mannermaa A, Kujala H, Laitinen T, Kosma V-M, Paajanen T, Kalviainen R, Hiltunen M, Herukka S-K, Karkkainen S, Kokkola T, Urjansson M, FinnGen, Perola M, Palotie A, Vuoksimaa E, Runz H. A FinnGen pilot clinical recall study for Alzheimer's disease. 2023 Feb 08 10.1101/2023.02.06.23285534 (version 1) medRxiv.
- Rodosthenous RS, Niemi ME, Kallio L, Perala M, Terho P, Knopp T, Punkka E, Makkonen EM, Nurmi P, Makela J, Wihuri P, Hautalahti M, Moffatt C, Martini P, Germine L, Makela VA, Karhunen OA, Lahti J, Hiekkalinna TS, Jyrhama T, Shen HY, Runz H, Palotie A, Perola M, Ganna A, FinnGen. Recontacting biobank participants to collect lifestyle, behavioural and cognitive information via online questionnaires: lessons from a pilot study within FinnGen. BMJ Open. 2022 Oct 5;12(10):e064695. PubMed.
- Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, Loukola A, Lahtela E, Mattsson H, Laiho P, Della Briotta Parolo P, Lehisto AA, Kanai M, Mars N, Rämö J, Kiiskinen T, Heyne HO, Veerapen K, Rüeger S, Lemmelä S, Zhou W, Ruotsalainen S, Pärn K, Hiekkalinna T, Koskelainen S, Paajanen T, Llorens V, Gracia-Tabuenca J, Siirtola H, Reis K, Elnahas AG, Sun B, Foley CN, Aalto-Setälä K, Alasoo K, Arvas M, Auro K, Biswas S, Bizaki-Vallaskangas A, Carpen O, Chen CY, Dada OA, Ding Z, Ehm MG, Eklund K, Färkkilä M, Finucane H, Ganna A, Ghazal A, Graham RR, Green EM, Hakanen A, Hautalahti M, Hedman ÅK, Hiltunen M, Hinttala R, Hovatta I, Hu X, Huertas-Vazquez A, Huilaja L, Hunkapiller J, Jacob H, Jensen JN, Joensuu H, John S, Julkunen V, Jung M, Junttila J, Kaarniranta K, Kähönen M, Kajanne R, Kallio L, Kälviäinen R, Kaprio J, FinnGen, Kerimov N, Kettunen J, Kilpeläinen E, Kilpi T, Klinger K, Kosma VM, Kuopio T, Kurra V, Laisk T, Laukkanen J, Lawless N, Liu A, Longerich S, Mägi R, Mäkelä J, Mäkitie A, Malarstig A, Mannermaa A, Maranville J, Matakidou A, Meretoja T, Mozaffari SV, Niemi ME, Niemi M, Niiranen T, O Donnell CJ, Obeidat ME, Okafo G, Ollila HM, Palomäki A, Palotie T, Partanen J, Paul DS, Pelkonen M, Pendergrass RK, Petrovski S, Pitkäranta A, Platt A, Pulford D, Punkka E, Pussinen P, Raghavan N, Rahimov F, Rajpal D, Renaud NA, Riley-Gillis B, Rodosthenous R, Saarentaus E, Salminen A, Salminen E, Salomaa V, Schleutker J, Serpi R, Shen HY, Siegel R, Silander K, Siltanen S, Soini S, Soininen H, Sul JH, Tachmazidou I, Tasanen K, Tienari P, Toppila-Salmi S, Tukiainen T, Tuomi T, Turunen JA, Ulirsch JC, Vaura F, Virolainen P, Waring J, Waterworth D, Yang R, Nelis M, Reigo A, Metspalu A, Milani L, Esko T, Fox C, Havulinna AS, Perola M, Ripatti S, Jalanko A, Laitinen T, Mäkelä TP, Plenge R, McCarthy M, Runz H, Daly MJ, Palotie A. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023 Jan;613(7944):508-518. Epub 2023 Jan 18 PubMed.
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012 Aug 2;488(7409):96-9. PubMed.
- Martiskainen H, Herukka SK, Stančáková A, Paananen J, Soininen H, Kuusisto J, Laakso M, Hiltunen M. Decreased plasma β-amyloid in the Alzheimer's disease APP A673T variant carriers. Ann Neurol. 2017 Jul;82(1):128-132. PubMed.
- Wittrahm R, Takalo M, Kuulasmaa T, Mäkinen PM, Mäkinen P, Končarević S, Fartzdinov V, Selzer S, Kokkola T, Antikainen L, Martiskainen H, Kemppainen S, Marttinen M, Jeskanen H, Rostalski H, Rahunen E, Kivipelto M, Ngandu T, Natunen T, Lambert JC, Tanzi RE, Kim DY, Rauramaa T, Herukka SK, Soininen H, Laakso M, Pike I, Leinonen V, Haapasalo A, Hiltunen M. Protective Alzheimer's disease-associated APP A673T variant predominantly decreases sAPPβ levels in cerebrospinal fluid and 2D/3D cell culture models. Neurobiol Dis. 2023 Jun 15;182:106140. Epub 2023 Apr 28 PubMed.
- Takalo M, Wittrahm R, Wefers B, Parhizkar S, Jokivarsi K, Kuulasmaa T, Mäkinen P, Martiskainen H, Wurst W, Xiang X, Marttinen M, Poutiainen P, Haapasalo A, Hiltunen M, Haass C. The Alzheimer's disease-associated protective Plcγ2-P522R variant promotes immune functions. Mol Neurodegener. 2020 Sep 11;15(1):52. PubMed.
- Natunen T, Martiskainen H, Marttinen M, Gabbouj S, Koivisto H, Kemppainen S, Kaipainen S, Takalo M, Svobodová H, Leppänen L, Kemiläinen B, Ryhänen S, Kuulasmaa T, Rahunen E, Juutinen S, Mäkinen P, Miettinen P, Rauramaa T, Pihlajamäki J, Haapasalo A, Leinonen V, Tanila H, Hiltunen M. Diabetic phenotype in mouse and humans reduces the number of microglia around β-amyloid plaques. Mol Neurodegener. 2020 Nov 10;15(1):66. PubMed.
- Diks AM, Teodosio C, de Mooij B, Groenland RJ, Naber BA, de Laat IF, Vloemans SA, Rohde S, de Jonge MI, Lorenz L, Horsten D, van Dongen JJ, Berkowska MA, Holstege H. Carriers of the p.P522R variant in PLCγ2 have a slightly more responsive immune system. Mol Neurodegener. 2023 Apr 20;18(1):25. PubMed.
- Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, Campos-Martin R, Grenier-Boley B, Andrade V, Holmans PA, Boland A, Damotte V, van der Lee SJ, Costa MR, Kuulasmaa T, Yang Q, de Rojas I, Bis JC, Yaqub A, Prokic I, Chapuis J, Ahmad S, Giedraitis V, Aarsland D, Garcia-Gonzalez P, Abdelnour C, Alarcón-Martín E, Alcolea D, Alegret M, Alvarez I, Álvarez V, Armstrong NJ, Tsolaki A, Antúnez C, Appollonio I, Arcaro M, Archetti S, Pastor AA, Arosio B, Athanasiu L, Bailly H, Banaj N, Baquero M, Barral S, Beiser A, Pastor AB, Below JE, Benchek P, Benussi L, Berr C, Besse C, Bessi V, Binetti G, Bizarro A, Blesa R, Boada M, Boerwinkle E, Borroni B, Boschi S, Bossù P, Bråthen G, Bressler J, Bresner C, Brodaty H, Brookes KJ, Brusco LI, Buiza-Rueda D, Bûrger K, Burholt V, Bush WS, Calero M, Cantwell LB, Chene G, Chung J, Cuccaro ML, Carracedo Á, Cecchetti R, Cervera-Carles L, Charbonnier C, Chen HH, Chillotti C, Ciccone S, Claassen JA, Clark C, Conti E, Corma-Gómez A, Costantini E, Custodero C, Daian D, Dalmasso MC, Daniele A, Dardiotis E, Dartigues JF, de Deyn PP, de Paiva Lopes K, de Witte LD, Debette S, Deckert J, Del Ser T, Denning N, DeStefano A, Dichgans M, Diehl-Schmid J, Diez-Fairen M, Rossi PD, Djurovic S, Duron E, Düzel E, Dufouil C, Eiriksdottir G, Engelborghs S, Escott-Price V, Espinosa A, Ewers M, Faber KM, Fabrizio T, Nielsen SF, Fardo DW, Farotti L, Fenoglio C, Fernández-Fuertes M, Ferrari R, Ferreira CB, Ferri E, Fin B, Fischer P, Fladby T, Fließbach K, Fongang B, Fornage M, Fortea J, Foroud TM, Fostinelli S, Fox NC, Franco-Macías E, Bullido MJ, Frank-García A, Froelich L, Fulton-Howard B, Galimberti D, García-Alberca JM, García-González P, Garcia-Madrona S, Garcia-Ribas G, Ghidoni R, Giegling I, Giorgio G, Goate AM, Goldhardt O, Gomez-Fonseca D, González-Pérez A, Graff C, Grande G, Green E, Grimmer T, Grünblatt E, Grunin M, Gudnason V, Guetta-Baranes T, Haapasalo A, Hadjigeorgiou G, Haines JL, Hamilton-Nelson KL, Hampel H, Hanon O, Hardy J, Hartmann AM, Hausner L, Harwood J, Heilmann-Heimbach S, Helisalmi S, Heneka MT, Hernández I, Herrmann MJ, Hoffmann P, Holmes C, Holstege H, Vilas RH, Hulsman M, Humphrey J, Biessels GJ, Jian X, Johansson C, Jun GR, Kastumata Y, Kauwe J, Kehoe PG, Kilander L, Ståhlbom AK, Kivipelto M, Koivisto A, Kornhuber J, Kosmidis MH, Kukull WA, Kuksa PP, Kunkle BW, Kuzma AB, Lage C, Laukka EJ, Launer L, Lauria A, Lee CY, Lehtisalo J, Lerch O, Lleó A, Longstreth W Jr, Lopez O, de Munain AL, Love S, Löwemark M, Luckcuck L, Lunetta KL, Ma Y, Macías J, MacLeod CA, Maier W, Mangialasche F, Spallazzi M, Marquié M, Marshall R, Martin ER, Montes AM, Rodríguez CM, Masullo C, Mayeux R, Mead S, Mecocci P, Medina M, Meggy A, Mehrabian S, Mendoza S, Menéndez-González M, Mir P, Moebus S, Mol M, Molina-Porcel L, Montrreal L, Morelli L, Moreno F, Morgan K, Mosley T, Nöthen MM, Muchnik C, Mukherjee S, Nacmias B, Ngandu T, Nicolas G, Nordestgaard BG, Olaso R, Orellana A, Orsini M, Ortega G, Padovani A, Paolo C, Papenberg G, Parnetti L, Pasquier F, Pastor P, Peloso G, Pérez-Cordón A, Pérez-Tur J, Pericard P, Peters O, Pijnenburg YA, Pineda JA, Piñol-Ripoll G, Pisanu C, Polak T, Popp J, Posthuma D, Priller J, Puerta R, Quenez O, Quintela I, Thomassen JQ, Rábano A, Rainero I, Rajabli F, Ramakers I, Real LM, Reinders MJ, Reitz C, Reyes-Dumeyer D, Ridge P, Riedel-Heller S, Riederer P, Roberto N, Rodriguez-Rodriguez E, Rongve A, Allende IR, Rosende-Roca M, Royo JL, Rubino E, Rujescu D, Sáez ME, Sakka P, Saltvedt I, Sanabria Á, Sánchez-Arjona MB, Sanchez-Garcia F, Juan PS, Sánchez-Valle R, Sando SB, Sarnowski C, Satizabal CL, Scamosci M, Scarmeas N, Scarpini E, Scheltens P, Scherbaum N, Scherer M, Schmid M, Schneider A, Schott JM, Selbæk G, Seripa D, Serrano M, Sha J, Shadrin AA, Skrobot O, Slifer S, Snijders GJ, Soininen H, Solfrizzi V, Solomon A, Song Y, Sorbi S, Sotolongo-Grau O, Spalletta G, Spottke A, Squassina A, Stordal E, Tartan JP, Tárraga L, Tesí N, Thalamuthu A, Thomas T, Tosto G, Traykov L, Tremolizzo L, Tybjærg-Hansen A, Uitterlinden A, Ullgren A, Ulstein I, Valero S, Valladares O, Broeckhoven CV, Vance J, Vardarajan BN, van der Lugt A, Dongen JV, van Rooij J, van Swieten J, Vandenberghe R, Verhey F, Vidal JS, Vogelgsang J, Vyhnalek M, Wagner M, Wallon D, Wang LS, Wang R, Weinhold L, Wiltfang J, Windle G, Woods B, Yannakoulia M, Zare H, Zhao Y, Zhang X, Zhu C, Zulaica M, EADB, GR@ACE, DEGESCO, EADI, GERAD, Demgene, FinnGen, ADGC, CHARGE, Farrer LA, Psaty BM, Ghanbari M, Raj T, Sachdev P, Mather K, Jessen F, Ikram MA, de Mendonça A, Hort J, Tsolaki M, Pericak-Vance MA, Amouyel P, Williams J, Frikke-Schmidt R, Clarimon J, Deleuze JF, Rossi G, Seshadri S, Andreassen OA, Ingelsson M, Hiltunen M, Sleegers K, Schellenberg GD, van Duijn CM, Sims R, van der Flier WM, Ruiz A, Ramirez A, Lambert JC. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022 Apr;54(4):412-436. Epub 2022 Apr 4 PubMed.
- Kämäläinen A, Viswanathan J, Natunen T, Helisalmi S, Kauppinen T, Pikkarainen M, Pursiheimo JP, Alafuzoff I, Kivipelto M, Haapasalo A, Soininen H, Herukka SK, Hiltunen M. GRN Variant rs5848 Reduces Plasma and Brain Levels of Granulin in Alzheimer's Disease Patients. J Alzheimers Dis. 2012 Aug 13; PubMed.
- Götzl JK, Brendel M, Werner G, Parhizkar S, Sebastian Monasor L, Kleinberger G, Colombo AV, Deussing M, Wagner M, Winkelmann J, Diehl-Schmid J, Levin J, Fellerer K, Reifschneider A, Bultmann S, Bartenstein P, Rominger A, Tahirovic S, Smith ST, Madore C, Butovsky O, Capell A, Haass C. Opposite microglial activation stages upon loss of PGRN or TREM2 result in reduced cerebral glucose metabolism. EMBO Mol Med. 2019 Jun;11(6) PubMed.
- Katsumata Y, Shade LM, Hohman TJ, Schneider JA, Bennett DA, Farfel JM, Alzheimer’s Disease Genetics Consortium, Kukull WA, Fardo DW, Nelson PT. Multiple gene variants linked to Alzheimer's-type clinical dementia via GWAS are also associated with non-Alzheimer's neuropathologic entities. Neurobiol Dis. 2022 Nov;174:105880. Epub 2022 Sep 30 PubMed.
- Korhonen VE, Remes AM, Helisalmi S, Rauramaa T, Sutela A, Vanninen R, Suhonen NM, Haapasalo A, Hiltunen M, Jääskeläinen JE, Soininen H, Koivisto AM, Leinonen V. Prevalence of C9ORF72 Expansion in a Large Series of Patients with Idiopathic Normal-Pressure Hydrocephalus. Dement Geriatr Cogn Disord. 2019;47(1-2):91-103. Epub 2019 Mar 12 PubMed.
- Rostalski H, Korhonen V, Kuulasmaa T, Solje E, Krüger J, Gen F, Kaivola K, Eide PK, Lambert JC, Julkunen V, Tienari PJ, Remes AM, Leinonen V, Hiltunen M, Haapasalo A. A Novel Genetic Marker for the C9orf72 Repeat Expansion in the Finnish Population. J Alzheimers Dis. 2021;83(3):1325-1332. PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.