Fresh Brain Every Friday: Biopsies Transform Alzheimer's Science
Quick Links
- A routine shunt surgery improves clinical care for people with hydrocephalus.
- Nearly half have Alzheimer's pathology, offering a natural model of LOAD.
- Every week, neurosurgeons supply electrophysiologists with cortex.
- They bank dura, CSF, blood, skin, and fat from the same patients, and follow them longitudinally.
- Fed into FinnGen, these samples enable functional studies on ADRD variants in APP, C9ORF72, GRN, TMEM106B, and other genes.
Heads up, Alzheimerologists around the world. This serialized report from Kuopio, a small Finnish city close to the Russian border, is well worth considering. It is a story about how tight—sometimes minute-by-minute—integration between medicine and science can benefit patients suffering from an obscure illness while simultaneously propelling neurodegeneration research to the next level. Together, Ville Leinonen, Tarja Malm, and Mikko Hiltunen—faculty in different disciplines at the University of Eastern Finland—have pioneered a system that might well inspire other medical research centers around the world. (Bicycles are involved.)

Where It Happens. Kuopio, in southeastern Finland, site of a unique brain biopsy cohort. [https://commons.wikimedia.org/wiki/File:Kuopio_aerial_6.jpg]
In this remote lakeside town ringed by birch forests, what happens to a person who can't walk anymore, wears diapers, and feels his or her mind fading because age-related hydrocephalus squashes their brain tissue? He or she can undergo a day surgery at Kuopio University Hospital that will restore their quality of life. During the procedure, a brain surgeon places a catheter through the frontal cortex at the top of the brain into the right ventricle. From there the catheter will drain the troublesome excess CSF into their abdominal cavity (image below).

The Basic Idea. One end of a silicon catheter is pushed through a burr hole in the skull above the right frontal cortex, and into the brain's ventricle. The other end is threaded under the skin to the abdominal cavity. This drains excess CSF from the brain, relieving the symptoms of chronic hydrocephalus. [Courtesy of Ville Leinonen.]
This procedure happens in many operating rooms around the world. But only in Kuopio does the surgeon collect up to eight different tissue samples along the way, including frontal cortex. If you count blood and CSF draws during the patient's pre-op and follow-up visits, the number of tissue samples from a single person exceeds a dozen. Many of those serve diagnostic purposes to better the patient's care, but they are also deposited in Finland's tissue banks for research. One—a bit of cortex—is studied within an hour of the surgery itself.
Together, the tissues and clinical-cognitive data collected from these patients enable discoveries across a range of different scientific methodologies, and from many organs, in hundreds of deeply phenotyped longitudinal study participants. Because idiopathic normal-pressure hydrocephalus (iNPH) afflicts people in their 60s, 70s, and 80s, these participants represent the spectrum of presymptomatic AD neuropathology. Indeed, about half have only NPH, others have amyloid deposits, yet others have plaques and tangles, or other causes of dementia. In short, the participants of this interwoven surgery-research program provide a natural model of early stage Alzheimer's disease and related disorders (ADRD).
Elsewhere in the world, brain biopsies are occasionally available to Alzheimer’s scientists at various research centers, typically when surgeons resect tumors or areas originating epileptic seizures. But it's not the same. In those diseases, surgeons avoid removing tissue that is functionally sound. Each biopsy tends to come from a different region, often from adults too young to be most informative for Alzheimer's research, and with varying amounts of attendant phenotypic information. In Kuopio, cerebro-ventricular biopsy collection is as standardized as the shunt surgery procedure itself. The cerebral biopsies all come from the same gyrus in area Brodmann area 8 of the right frontal cortex. Every week, the same team of neurosurgeons collects, studies, and preserves the samples in exactly the same way.
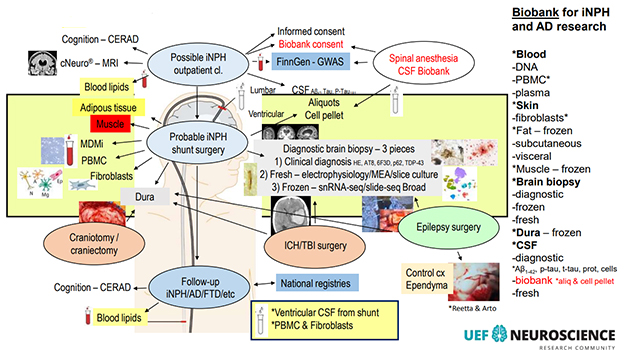
Lots Going On. This busy diagram lists most of the procedures in, and some research uses of, the Kuopio iNPH shunt sampling protocol. [Courtesy of V. Leinonen, KUH.]
One of the samples is unique in the world. It is a pyramid-shaped piece gently lifted from the top of the cortex where the shunt catheter will enter, and it is studied on the same day while still “alive.” No other lab does this. At 3 mm per side, the pyramid is only as wide as the silicon tube—the shunt—that will go into the cortex in its place. That is tiny compared to the occasional epilepsy or tumor resection biopsy a lucky lab scientist may get their hands on. Even so, this pyramid is big enough to encompass a "working unit" of cerebral cortex (image below). From a square of pia mater at its top, it reaches down through layers 1 to 6, to a tip of white matter at its bottom (see Part 2 of this series.)
Within five minutes of coming out of the patient, the pyramid leaves the operating room, lands in the hands of an electrophysiologist waiting by the hospital door, and rides by bicycle to a research building 0.8 miles down the hill. There, serial slices cut from it soon find themselves mounted in two recording rigs—one a single-cell patch clamp, the other a multi-electrode array. For the next eight hours or so, neurons in these acute human brain slices fire, burst, and oscillate—all while their donor up on the hill comes to in the recovery room, and gets ready to go home (for details, see Part 3 of this series).

From Brain to Vibratome in a Hurry. Within an hour of coming out, a pyramid-shaped piece of frontal cortex is sliced, recorded from, and preserved for other methods of study. [Courtesy of Henna Jäntti].
All tissues collected during these weekly ventriculo-peritoneal shunt placements form part of the most comprehensive, collaborative ADRD biopsy research protocol in the world. Beyond the cortical pyramid, it has many additional components.
For example, the neurosurgeon, with a punch needle, pulls two even tinier bits of cortex from where the pyramid just came out and slides them into liquid nitrogen. They are shipped to the labs of Evan Macosko and Beth Stevens at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. There, scientists profile the gene expression signatures of each cortical cell type and run multi-omics studies feasible only with fresh frozen brain.
A first major manuscript from this ongoing work by the Broad/UEF collaborators appeared on June 5 on bioRXiv. It characterizes, in the brains of living people, early cellular changes across three stages of increasing AD pathology. In a nutshell, the loss of a particular type of layer 1 inhibitory neuron appears to render a particular type of layer 2/3 excitatory neuron hyperactive. These neurons fade soon after, while microglial subpopulations shift toward neuroinflammatory states. The Aβ instigating this slow-moving calamity comes not only from the neurons themselves, but also from oligodendrocytes and their interface with axons of the doomed pyramidal neurons, drawing attention to myelin in early AD (Gazestani et al., 2023; Part 4 of this series).
The Broad also receives blood from the same patients for whole-genome sequencing. Other vials of blood stay at the University of Eastern Finland. One is used for the patient's diagnosis and follow-up care; one goes to Hiltunen's lab for studies exploring its monocytes; others are frozen and banked for proteomic, metabolomic, and lipidomic analyses with collaborators at UEF and elsewhere.
A sliver of skin is taken—not separately via punch biopsy as is customary in research, but right as the surgeon opens the abdomen to situate the end of the shunt catheter descending, underneath the patient's skin, from his or her brain. This skin sample will yield fibroblasts that are reprogrammed to iPSCs, then differentiated into various cell types for research use. This happens in Malm's lab at UEF's A.I. Virtanen Institute for Molecular Sciences.
Four other types of tissue from the patients have been banked at UEF for some years. Biding their time in a freezer, they are awaiting the right research question to emerge for projects either at UEF or in collaboration with other universities. They are: lumps of fat from under the skin; if present, lumps of fat from the peritoneal cavity near where the catheter ends; a morsel of muscle from the abdominal wall; and slivers of dura for research on the brain's border tissues.
CSF is taken often, and copiously. After all, having too much of it is part of the problem for people with NPH. When a person whose symptoms could be due to NPH gets referred to Kuopio University Hospital, a spinal tap drawing 40 ml is part of the diagnostic workup. That's because if the patient’s gait improves after such a large reduction in CSF volume, it is a hint NPH may be the underlying etiology. Adult humans have, on average, 120 to 150 ml of CSF. Confirmation of the NPH diagnosis requires a subsequent MRI.
During the shunt surgery, nurses collect up to 20 ml of CSF directly from the right ventricle the moment the shunt tip enters. This relieves pressure in the patient's brain, accelerating symptom relief. It also gives researchers an opportunity to compare the CSF deep inside a person's brain with CSF in the spinal canal. One such study quantified the concordance of ventricular and lumbar CSF Aβ42, total tau, and p-tau 181 in 138 NPH patients (Lukkarinen et al., 2023).
The Kuopio protocol calls for the surgeon and her nurses to collect the first few drops coming out of the freshly shunted ventricle in a separate tube from the bulk of the ventricular fluid volume. Why? This initial drip contains cortical cells shorn off as the shunt catheter penetrates through the gyrus into the ventricle. These cells are spun down and cryoprotected, so that their transcriptomes can be studied separately from those of the immune cells more typically found in CSF.
What's in it for the patient?
The data being generated from this range of tissues exist in a rich context thanks to detailed phenotypic and clinical information being gathered on each participant. After the surgery, patients return to the clinic for many follow-up visits, both to improve their care and for research. At the first post-op, doctors optimize the shunt's flow via a small valve implanted under the skin behind the ear. Later visits are annual checkups if the shunt works fine, or “repair” visits should it get blocked. Each time, if malfunction is suspected, a minor skin puncture to access the valve generates yet another sample of ventricular fluid for longitudinal research. Most of it gets banked at UEF; aliquots go to Sweden's University of Gothenburg as part of a long-standing proteomics and biomarker collaboration with Kaj Blennow and Henrik Zetterberg.
The cortex pyramid, too, serves multiple purposes beyond satisfying the curiosity of electrophysiologists. All but the two serial slices that are used in the live recordings are prepped for other uses. One gets frozen for Slide-Seq spatial transcriptomics of the intact cellular architecture of aging human cortex. One gets fixed and sliced into thin sections for research immunohistochemistry, and one is for electron microcopy. Any extra bits go toward proteomic studies. Those are research uses.
One slice of the pyramid goes back up the hill to the university hospital, where neuropathologist Tuomas Rauramaa stains them for routine clinical diagnostic markers. Using antibodies against pathological forms of Aβ, tau, α-synuclein, TDP-43, as well as markers of Trem2/microglial activation and the autophagy marker p62, Rauramaa's team generates molecular information to sharpen the shunt recipient's diagnosis. Knowing whether a patient has “pure” hydrocephalus, or also a concomitant neurodegenerative process, informs their follow-up care.
In Kuopio, diagnostic use of biopsies in longitudinally followed shunt patients started more than 30 years ago, hence scientists today know more about how NPH overlaps with age-related neurodegenerative diseases. For example, amyloid positivity is more frequent among people with NPH than in healthy controls, perhaps because an underlying drainage problem accelerates accumulation of Aβ (e.g., Luikku et al., 2019). Even so, most patients' NPH symptoms improve for a while after the shunt procedure, regardless of whether they have plaques, or plaques and tangles. For this reason, all patients with a confirmed NPH diagnosis are candidates for shunt surgery in Kuopio, even those with concomitant neurodegenerative disease. Typically, the latter benefit for fewer years than those with “pure” NPH, as the shunt does not treat the other illnesses. The mean survival time after shunt placement is five years, according to Leinonen.
How Much Does the Operation Help?
Up to 90 percent of shunt recipients can cast aside their walker or wheelchair, and regain control of their bladder, Leinonen told Alzforum. Regarding cognition, people with MCI before surgery tend to revert to normal; those with mild dementia improve to MCI and regain independence. Surgeons at five centers across the U.S. confirmed that this treatment is safe and effective (Williams et al., 2022), and a Swedish study found that it prolongs life (Andrén et al., 2021). A multicenter RCT is ongoing in the U.S.
But it is not a permanent solution. One study reported that only a third of recipients are still better by three years post-op (Espay et al., 2017). To this cautionary finding, the Kuopio and Gothenburg teams responded by searching for biomarkers that might predict shunt response. On June 5, they report that an unbiased tandem mass tag proteomic analysis of 68 shunt recipients' pre-operative CSF netted nine candidate markers (Weiner et al., 2023).
A person's longer-term cognitive outcome depends on whether (s)he has another neurodegenerative disease. Appearances, however, can be deceiving. For example, Leinonen recalled a man whose MMSE had risen post-shunt to 29/30, but over the next 2.5 years gradually sank again, to 19. "He had amyloid in his biopsy, so we thought now it may be the AD taking over," Leinonen told Alzforum. "But we checked his shunt and found it was blocked. After that was cleared, his MMSE went back up to 26 after two months, and he functioned quite normally again." This underscores the importance of follow-up care: "When our patients worsen, we often can get them back on track," he said (image below).
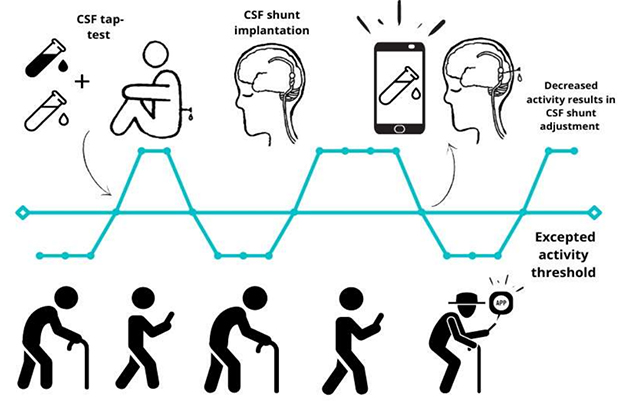
Track That Shunt. During follow-up care, adjustments to the shunt's flow can restore its treatment benefit, while also generating longitudinal CSF samples. In the future, digital monitoring may detect when the shunt malfunctions. [Courtesy of Ville Leinonen and Rosa Sahlström, KUH.]
For the treating physicians, having data on both cortical tissue and CSF paints a more complete picture of the patient in front of them. Ditto for their likely prognosis. Alas, partly because NPH often overlaps with other diseases of aging, NPH is not treated aggressively in many clinics around the world. Without a molecular diagnosis, this murkiness can feed nihilism among neurologists, who may think: "Why subject this person to surgery when they probably have some untreatable dementia?" In contrast, the Kuopio clinician-researchers see this overlap as an opportunity: "We can make this person better while studying both their NPH and their preclinical Alzheimer's or FTD," Leinonen said.
The risk of overtreatment is small, he believes, because MRI easily rules out hydrocephalus. The condition grossly distends the ventricles, creating dramatic, fluid-filled caverns where brain tissue should be. NPH also flattens the distinctive folds of gyri and sulci in areas where the cortex gets pressed against the skull (image below).

Too Much Pressure. MRI scans of chronic hydrocephalus. The disease enlarges the brain's ventricles, creates CSF-filled cavities, and can flatten cortical gyri. The right image represents a subgroup called LOVA. [Courtesy V. Leinonen].
The diagnostic benefit of the brain and CSF biopsies explains why the Kuopio sampling protocol comports with medical ethics regulations. In Finland, these are partially based on the Declaration of Helsinki and are as rigorous as in any nation in the world. Because diagnostic biopsies are an integral part of patient care, a generic “umbrella” consent for their additional research use suffices to support this highly differentiated hydrocephalus shunt protocol. The sampling does not increase patients' risk during surgery, unlike trials of experimental therapies, which do come with new and unknown risks.
Most patients consent readily. "Finnish people are positively inclined toward research," Leinonen told Alzforum. In the rare cases when a patient declines research use of their shunt biopsies, the scientists take no skin, fat, or muscle, and a smaller volume of blood. They still use a cortical biopsy and CSF for diagnosis, but do not bank it.
Surprisingly, perhaps, removing a small volume of cortical tissue causes no known cognitive loss. In humans, no specific function has yet been discovered for the gyrus in question. That is why Brodmann 8 serves as a standard point of entry when trauma neurosurgeons have to “go in” to lower intracranial pressure, or drain fluid, in emergency situations, such as brain trauma after an accident, or subarachnoid hemorrhage after a stroke or aneurysm.
Who set up this sampling protocol? The lead instigator is Leinonen, a neurosurgeon at UEF. A spinal neurosurgeon by specialty, Leinonen says he thinks about research all the time. His interest was kindled when, as a resident and postdoc, he used hydrocephalus frontal cortex biopsy tissue to validate a then-brand-new amyloid PET tracer called PIB (Leinonen et al., 2008). "That was my first experience with Alzheimer's research," he said.

Doing Well While Doing Good With Biopsies? Ville Leinonen at Kuopio University Hospital, holding a Dewar that will snap freeze bits of cortex and dura the minute they are taken from the human brain, for RNA-Seq and other analyses at the Broad Institute in Cambridge, Massachusetts. [Image courtesy of Gabrielle Strobel.]
A key partner is Malm. A molecular biologist, she runs a 25-person lab. Every week for six months in 2020, Antonios Dougalis, a neurophysiologist in Malm's lab, conferred with Leinonen's team of neurosurgeons as he tried to establish recordings from the biopsies. Dougalis realized that conventional ways of sampling brain tissue—sucking out a worm-shaped piece with a syringe needle or grabbing a bit with biopsy forceps—squeezed the neurons inside such that they would not fire. This prompted Antti Luikku, a resident with Leinonen, to cut four pyramid sides with the tip of a surgery knife and lift it out ever so delicately on the knife's flat side. Other scientists in Malm's lab are making iPSC-derived cell types and organoids from skin biopsies of interest, particularly from shunt patients who happen to carry genetic variants sought after for mechanistic studies, such as in progranulin and other genes.
Last but not least, the whole operation is part of UEF's larger Neuroscience Research Community, an organizational and funding framework overseen by Hiltunen, a molecular geneticist.
In 2013, Hiltunen and Leinonen began extracting RNA from iNPH cortex biopsies. They learned then that they needed to flash-freeze the tissue right in the operating room if they wanted the RNA to stay intact for sequencing. From there, Hiltunen and Leinonen continued building a sampling program. Today, Mari Takalo in Hiltunen's lab runs research projects that use NPH biopsy tissues for functional genomics and personalized medicine studies. She currently focuses on variants in PLCG2, progranulin, Abi3, APP, TREM2, TyroBP—all of which occur among Kuopio's NPH shunt recipients. Scientists led by UEF molecular biologist Annakaisa Haapasalo study C9ORF72. Hexanucleotide expansions in this gene are Finland's most common cause of FTD—and are found in the NPH shunt cohort (for more, see Part 5 of this series).
A Look Back in Time
The Kuopio biopsy research protocol evolved over the past three decades. At KUH, the practice of improving hydrocephalus shunt placement with biopsies dates back to 1991, when the late neurosurgeon Matti Vapalahti trained his resident Sakari Savolainen, who made the combination routine. An early champion through the 1990s and 2000s was Irina Alafuzoff, a prominent ADRD neuropathologist who worked in Kuopio before moving to Sweden's Uppsala University. Another was neurosurgeon Juha Jääskeläinen, who forged the connection between NPH and AD research when Leinonen was a resident, starting in 2005.
In 2010, the KUH neurologist Anne Koivisto explored the link between NPH and AD. She retrospectively analyzed brain biopsies stained for Aβ and tau going back 20 years. Koivisto screened intake records from 468 NPH patients to match cognitive decline noted in them against diagnostic neuropathology criteria for AD as seen in their cortices. This led to the realization that nearly half of shunted NPH patients—including those who after the shunt get better for several years—develop dementia eventually. Mostly, they were the ones who had AD pathology, followed by those with vascular pathology (Leinonen et al., 2010). In the 13 years since, postmortem neuropathology findings of those early patients, plus biopsy data from more recent ones, have made clear that about one in 100 people with NPH also have multiple-system atrophy, Lewy body dementia, corticobasal degeneration, or another frontotemporal dementia, Leinonen told Alzforum.
The older components of the Kuopio NPH biopsy protocol—cortex, CSF, CSF cells, blood, skin—have been banked for many years such that samples and cell lines are available on several hundred patients. Over the years, the protocol has become standardized.
It also has grown, both in terms of how many shunts are placed each year, and how much information is being collected on each patient. Since acute slice electrophysiology started in 2020, preoperative fMRI was added in June 2022. Currently, scientists are working out how to also collect a tiny drop of CSF from the arachnoid space right above the brain in hopes of enabling research on these border layers. Also under consideration: adding preoperative EEG to relate the results of acute cortical slice recordings, i.e., local activity, with regional brain activity.
Nowadays, 50 NPH shunts are placed at KUH annually by a team of brain surgeons. It is a routine procedure, performed by residents on most Fridays and some Tuesdays. NPH shunt placements in the country's other four university hospitals—Helsinki, Oulu, Tampere, and Turku—bring the annual number in all of Finland to 150, according to Leinonen, though not all sites collect research biopsies comprehensively.
Some other centers in Scandinavian countries take and study NPH shunt biopsies. Research on NPH itself occurs at Rikshospitalet in Oslo, most recently with a study visualizing the fragile meningeal lymphatic vessels in dura mater (Vera Quesada et al., 2023). ADRD fluid-based biomarker and proteomics science with NPH CSF happens at UGothenburg, and tissue neuropathology research at University of Uppsala, from where Alafuzoff recently retired. Throughout Europe and the U.S., shunts are available as a treatment for hydrocephalus, but these sites do not routinely collect and bank biopsy tissue as part of an ongoing ADRD clinical research program.
Some one-off studies exist. For example, in New York City, Columbia University neurosurgeon Guy McKhann and neuropathologist Andrew Teich published bulk RNA-Seq analysis of NPH cortical biopsies, described gene expression patterns indicating a microglial state change when there was concomitant AD pathology (Huang et al., 2021). At Johns Hopkins University in Baltimore, researchers used NPH shunt biopsies to validate the amyloid PET tracer flutemetamol (Wong et al., 2013).
Leinonen and colleagues contribute their data on the cohort of NPH shunt patients—more than 1,000 going back to 1993—to FinnGen, the country's equivalent of the U.K. Biobank, for DNA genotyping and data integration. FinnGen serves as the national hub for de-identified clinical data on the NPH patients' clinic visits, hospitalizations, surgeries, prescriptions, and causes of death for those who have died. The FinnGen dataset amounts to searchable lifelines of these people alongside similar data on soon-to-be 500,000 other Finns as controls (see Part 5).
The Kuopio researchers collaborate on these biopsy projects with colleagues in Norway, Sweden, Germany, and the U.S. If more hospitals that place those shunts built similar tissue collection protocols, studies could combine forces to achieve larger group sizes, especially on the biology of rare gene variants. Age-related hydrocephalus is, after all, itself a relatively rare disease. Leinonen told Alzforum that the Kuopio team would be happy to teach visiting surgeons and neurophysiologists interested in starting similar programs at their universities. (Parts 2, 3, 4, 5).—Gabrielle Strobel
References
News Citations
- A Day's Work: Cortex Biopsy Comes Out. Shunt Goes In. Patient Goes Home.
- Brain Tissue From Living People with Amyloid Plaques Can Fire in a Dish
- Cortical Biopsies Hint at Start of Alzheimer's 'Cellular Phase'
- Brain Biopsies and FinnGen Form Wellspring for Functional Genomics
Paper Citations
- Gazestani VH, Kamath T, Nadaf NM, Burris S, Rooney B, Junkkari A, Vanderburg C, Rauramaa T, Therrien M, Tegtmeyer M, Herukka S-K, Abdulraouf A, Marsh S, Malm T, Hiltunen M, Nehme R, Stevens B, Leinonen V, Macosko EZ. Early Alzheimer's disease pathology in human cortex is associated with a transient phase of distinct cell states. 2023 Jun 05 10.1101/2023.06.03.543569 (version 1) bioRxiv.
- Lukkarinen H, Vanninen A, Tesseur I, Pemberton D, Van Der Ark P, Kokkola T, Herukka SK, Rauramaa T, Hiltunen M, Blennow K, Zetterberg H, Leinonen V. Concordance of Alzheimer's Disease-Related Biomarkers Between Intraventricular and Lumbar Cerebrospinal Fluid in Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2023;91(1):305-319. PubMed.
- Luikku AJ, Hall A, Nerg O, Koivisto AM, Hiltunen M, Helisalmi S, Herukka SK, Junkkari A, Sutela A, Kojoukhova M, Korhonen V, Mattila J, Lötjönen J, Rummukainen J, Alafuzoff I, Jääskeläinen JE, Remes AM, Solomon A, Kivipelto M, Soininen H, Rauramaa T, Leinonen V. Predicting Development of Alzheimer's Disease in Patients with Shunted Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2019;71(4):1233-1243. PubMed.
- Williams MA, Nagel SJ, Golomb J, Jensen H, Dasher NA, Holubkov R, Edwards RJ, Luciano MG, Zwimpfer TJ, Katzen H, Moghekar A, Wisoff JH, McKhann GM, Hamilton MG. Safety and effectiveness of the assessment and treatment of idiopathic normal pressure hydrocephalus in the Adult Hydrocephalus Clinical Research Network. J Neurosurg. 2022 Mar 11;:1-13. PubMed.
- Andrén K, Wikkelsø C, Hellström P, Tullberg M, Jaraj D. Early shunt surgery improves survival in idiopathic normal pressure hydrocephalus. Eur J Neurol. 2021 Apr;28(4):1153-1159. Epub 2021 Jan 25 PubMed.
- Espay AJ, Da Prat GA, Dwivedi AK, Rodriguez-Porcel F, Vaughan JE, Rosso M, Devoto JL, Duker AP, Masellis M, Smith CD, Mandybur GT, Merola A, Lang AE. Deconstructing normal pressure hydrocephalus: Ventriculomegaly as early sign of neurodegeneration. Ann Neurol. 2017 Oct;82(4):503-513. Epub 2017 Oct 4 PubMed.
- Weiner S, Junkkari A, Sauer M, Luikku A, Rauramaa T, Kokkola T, Herukka SK, Blennow K, Zetterberg H, Leinonen V, Gobom J. Novel cerebrospinal fluid biomarkers correlating with shunt responsiveness in patients with idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2023 Jun 5;20(1):40. PubMed.
- Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Någren K, Tapiola T, Pirttilä T, Rinne J, Jääskeläinen JE, Soininen H, Rinne JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008 Oct;65(10):1304-9. PubMed.
- Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, Vainikka S, Pyykkö OT, Mölsä J, Fraunberg M, Pirttilä T, Jääskeläinen JE, Soininen H, Rinne J, Alafuzoff I. Amyloid and tau proteins in cortical brain biopsy and Alzheimer's disease. Ann Neurol. 2010 Oct;68(4):446-53. PubMed.
- Vera Quesada CL, Rao SB, Torp R, Eide PK. Immunohistochemical visualization of lymphatic vessels in human dura mater: methodological perspectives. Fluids Barriers CNS. 2023 Mar 28;20(1):23. PubMed.
- Huang W, Bartosch AM, Xiao H, Maji S, Youth EH, Flowers X, Leskinen S, Tomljanovic Z, Iodice G, Boyett D, Spinazzi E, Menon V, McGovern RA, McKhann GM, Teich AF. An immune response characterizes early Alzheimer's disease pathology and subjective cognitive impairment in hydrocephalus biopsies. Nat Commun. 2021 Sep 27;12(1):5659. PubMed.
- Wong DF, Moghekar AR, Rigamonti D, Brašić JR, Rousset O, Willis W, Buckley C, Smith A, Gok B, Sherwin P, Grachev ID. An in vivo evaluation of cerebral cortical amyloid with [18F]flutemetamol using positron emission tomography compared with parietal biopsy samples in living normal pressure hydrocephalus patients. Mol Imaging Biol. 2013 Apr;15(2):230-7. PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Rowan University, NJ Institute for Successful Aging
Shunting for NPH is a routine “bread-and-butter” neurosurgical procedure which all neurosurgeons in the U.S.A. have done at some point in their careers, myself included.
Decades of clinical experience have shown that it often works fairly well to improve the “magnetic gait” of NPH and may help to some extent with urinary frequency symptoms. However, most of the time it has very minimal effects on cognitive symptoms.
This project is certainly interesting, but performing clinically unnecessary brain biopsies during a routine neurosurgical procedure is not something most IRB’s in the U.S.A. would look favorably upon. It will be interesting to see which biomarkers they find are most predictive of good response to therapy.
Medical University Vienna
For a neuropathologist who has worked for more than five decades, it is satisfying to read this well-written article about the standardized research that is done at KUH, pioneered by Irina Alafuzoff a long time ago. Taking brain biopsies routinely during NPH shunting is nothing new, and NPH is not AD, but the KUH approach excels by the breadth and multidisciplinarity of its sampling protocol, something that is the very basis of promising top-class tissue studies. I disagree with Dr. Jason's comment that these brain biopsies are unnecessary; the article also gives good citations why this is untrue. It is this type of human tissue research that will bring us forward in our understanding of the puzzle of neurodegeneration.
Co-Director, Brigham and Women's Hospital's Ann Romney Center for Neurologic Diseases
Those of us in biomedical research become excited about the advent of highly sophisticated new technology applicable to studies we wish to accelerate. We don’t commonly think of scientific novelty emerging from basic procedures that have been used for many decades, for example, clinicopathological correlation. Gabrielle Strobel’s illuminating articles remind us of the unique, long-running NPH/brain biopsy/fluid+tissue sampling program at Kuopio University Hospital (KUH). The Alzforum series describes a thoughtful and efficient coming together of well-established procedures such as shunt placement for NPH, cortical biopsies for diagnostic and quantitative neuropathology, and broad correlations with both omics and fluid biomarkers to better understand the patterns of neurodegenerative lesions and altered CSF dynamics in some elderly humans.
In my view, taking advantage of the need for shunt placement in the right NPH patients evaluated as described herein and obtaining informed consent for cortical biopsies that can contribute to diagnostic and prognostic accuracy, while simultaneously enabling novel human research, is highly worthwhile if done under careful ethical guidance. Not all countries and certainly not all neurosurgeons and allied clinicians will favor this pro-research approach, but the KUF experience shows that it can be done appropriately, with informative scientific clarifications being achieved. This articulate summary may inspire additional neurosurgery, neurology, and basic research groups studying age-related brain disease to establish similar programs. I suspect Drs. Leinonen, Malm, and Hiltunen would be happy to advise fellow specialists in NPH, AD, and FTD (among other disorders) about how to create an efficient and ethical program like theirs.
In neurology, we know that “pure” idiopathic NPH often arises in people who have had a distant history of intracerebral bleeding or meningitis, which can slowly compromise facile CSF circulation. However, other patients may have NPH with no such predisposing factors, and the KUF group and others have shown us that many patients with NPH also have neurodegenerative lesions, especially the plaques and tangles of AD. This reality can allow us to observe during life the lesions of AD and the associated cellular, protein, and lipid changes which accompany the complex Alzheimer syndrome. As such, accessing living tissue for electrophysiology, omics analyses, and detailed immunohistochemical probing can and has advanced our understanding of neurodegenerative disease of the cerebral cortex.
One other thought: The direct visualization of AD histopathology and simultaneous CSF biomarkers as contributing to a patient’s diagnosis emerges in a new light when one considers how this can facilitate the entry of a few more AD patients into disease-modifying anti-amyloid immunotherapy. While brain biopsy has long been rightly considered as unnecessary for AD diagnosis, the fact that it can be easily and safely performed if a patient needs shunting for NPH means that a program like KUF’s may identify patients who are appropriate for amyloid-lowering therapies, assuming they meet the other criteria (e.g., Cummings et al., 2023).
References:
Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2023. Alzheimers Dement (N Y). 2023;9(2):e12385. Epub 2023 May 25 PubMed. Correction.
Columbia University
This is a fantastic article highlighting the impressive work being done by the combined research and clinical team in Finland. At our own NPH clinic at Columbia University/New York Presbyterian Hospital we are doing similar/complementary studies to what is described here in a multidisciplinary collaborative neuroscience setting. We have routinely done brain biopsies and CSF collection as part of our shunt surgeries for the past decade. At our center, biopsy tissue is only taken at the shunt insertion point, and has diagnostic and prognostic value in the clinical care of these patients. We think this is a patient cohort that has a great deal to contribute to our understanding of AD, and hope other centers are able to contribute to this emerging avenue of research.
—Guy M. McKhann II, M.D., Columbia University, is a co-author of this comment.
DZNE
This series elegantly highlights remarkable research on precious live human brain tissue possible only through the close collaboration of clinicians and researchers. Ville Leinonen, Tarja Malm, and Mikko Hiltunen have established a very powerful example of combining routine clinical procedures with disease-motivated basic research. In our experience, the vast majority of patients in fact support such an approach. They provide informed consent enabling collection of various tissues and fluids (blood, CSF, resected brain tissue, and biopsies) accrued during a medically indicated surgical procedure. Taking advantage of these precious resources in the most optimal way, through streamlining and meticulous planning of the entire procedure from the operating room to the laboratory, now facilitates new, unprecedented discoveries (see Gazestani et al., 2023).
The Finnish group uses a multimodal approach to investigate the resected brain samples, ranging from state-of-the-art techniques to analyze the function of neurons using patch clamp and MEA recordings to proteomics and spatial transcriptomics. The value and preciousness of the human (brain) tissue require that we push the limit with this finite material to investigate the human brain’s physiological and pathophysiological properties.
Along this line, we and others have utilized human organotypic brain slice cultures from cortical tissue resected due to epilepsy and/or glioma. This has extended the window for investigating human brain physiology in vitro (Eugène et al., 2014; Schwarz et al., 2017, 2019; Lee et al., 2022) and for being able to examine the progression of neurodegenerative disease pathology (Barth et al, 2021).
Combining these approaches to investigate the human brain at the cellular level will enable us to gain much deeper insight into the mechanisms of aging and age-related neurodegenerative diseases, and funders are seeing the value of this approach.
—Thomas Wuttke is a neurosurgeon and clinical researcher at the Hertie Institute for Clinical Brain Research, Henner Koch is a neurophysiologist at RWTH Aachen Medical School; both are co-authors of this comment.
References:
Barth M, Bacioglu M, Schwarz N, Novotny R, Brandes J, Welzer M, Mazzitelli S, Häsler LM, Schweighauser M, Wuttke TV, Kronenberg-Versteeg D, Fog K, Ambjørn M, Alik A, Melki R, Kahle PJ, Shimshek DR, Koch H, Jucker M, Tanriöver G. Microglial inclusions and neurofilament light chain release follow neuronal α-synuclein lesions in long-term brain slice cultures. Mol Neurodegener. 2021 Aug 11;16(1):54. PubMed.
Eugène E, Cluzeaud F, Cifuentes-Diaz C, Fricker D, Le Duigou C, Clemenceau S, Baulac M, Poncer JC, Miles R. An organotypic brain slice preparation from adult patients with temporal lobe epilepsy. J Neurosci Methods. 2014 Sep 30;235:234-44. Epub 2014 Jul 23 PubMed.
Gazestani V, Kamath T, Nadaf NM, Burris SJ, Rooney B, Junkkari A, Vanderburg C, Rauramaa T, Therrien M, Tegtmeyer M, Herukka SK, Abdulraouf A, Marsh S, Malm T, Hiltunen M, Nehme R, Stevens B, Leinonen V, Macosko EZ. Early Alzheimer's disease pathology in human cortex is associated with a transient phase of distinct cell states. bioRxiv. 2023 Jun 5; PubMed.
Lee B, Dalley R, Miller JA, Chartrand T, Close J, Mann R, Mukora A, Ng L, Alfiler L, Baker K, Bertagnolli D, Brouner K, Casper T, Csajbok E, Dee N, Donadio N, Driessens SL, Egdorf T, Enstrom R, Galakhova AA, Gary A, Gelfand E, Goldy J, Hadley K, Heistek TS, Hill D, Johansen N, Jorstad N, Kim L, Kocsis AK, Kruse L, Kunst M, Leon G, Long B, Mallory M, Maxwell M, McGraw M, McMillen D, Melief EJ, Molnar G, Mortrud MT, Newman D, Nyhus J, Opitz-Araya X, Pham T, Pom A, Potekhina L, Rajanbabu. Signature morpho-electric properties of diverse GABAergic interneurons in the human neocortex. 2022 Nov 09 10.1101/2022.11.08.515739 (version 1) bioRxiv.
Schwarz N, Uysal B, Welzer M, Bahr JC, Layer N, Löffler H, Stanaitis K, Pa H, Weber YG, Hedrich UB, Honegger JB, Skodras A, Becker AJ, Wuttke TV, Koch H. Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease. Elife. 2019 Sep 9;8 PubMed.
Schwarz N, Hedrich UB, Schwarz H, P A H, Dammeier N, Auffenberg E, Bedogni F, Honegger JB, Lerche H, Wuttke TV, Koch H. Human Cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures. Sci Rep. 2017 Sep 25;7(1):12249. PubMed.
Ludwig-Maximilians-Universität Munich
I fully agree with my colleague Herbert Budka on this very well-written article on the usefulness of brain biopsies—and not only in the context of NPH. In times where AD becomes treatable if the diagnosis is made early, we have to rethink our concerns taking a very small cortical biopsy in the context of any kind of brain surgery in patients above 60 years of age and at higher risk to develop AD (for example ApoE4 positive). Why not get an idea if AD is in a presymptomatic stage if the biopsy is not harmful?
Moreover, the management of elderly people with diseases that require an operation on the brain may be different if the analysis of the brain tissue reveals high amyloid and tau load. In fact, I find it problematic if the operation of subdural or intracerebral bleeding in older people does not include a small cortical biopsy to exclude CAA or strong presymptomatic AD pathology. There are occasions where chronic subdural hematoma in old people appears clinically as dementia. If after the removal of the clotted blood, cognitive symptoms do not improve, further diagnostic is needed, which could be easily avoided if a cortical biopsy had been taken.
Make a Comment
To make a comment you must login or register.