Gut Microbiome May Modify Neurodegeneration
Quick Links
At the Society for Neuroscience annual meeting, held November 11–15 in Washington, D.C., scientists presented further hints that bacteria in the gut may influence the symptoms and pathology of Alzheimer’s and other neurodegenerative diseases. Tweaking the bacterial profile in the digestive tracts of transgenic mice—either by feeding them probiotics or supplementing wild-type microbiota—reduced amyloid plaques, soothed inflammation, and improved memory. ApoE genotype correlated with different species of bacteria in the mouse gut, and certain bacterial metabolites prevented misfolded proteins from aggregating. Together, these studies showed how researchers are tackling the nexus between the microbiome and neurodegeneration.
- Adjusting the composition of bacteria in the gut may influence Alzheimer’s.
- Doing so altered memory, pathology, and inflammation in mice.
- Bacterial byproducts could influence aggregation and apoptosis.
“This area of research is set to explode in the next few years,” said Dave Morgan, now at Michigan State University College of Human Medicine in Grand Rapids.
Suzana Petanceska, National Institute on Aging, agreed. “Microbiome research is not just an emerging, but an expanding area of research into a variety of disease areas—from obesity and cancer to neurodevelopmental and neuropsychiatric disorders," she said.
Over the past two years, scientists have reported that some species in the microbiome can promote protein aggregation in certain neurodegenerative diseases, including Parkinson’s and Alzheimer’s (Dec 2016 news). Gut microbes seem to exacerbate Aβ pathology in mouse models of amyloidosis, and obliterating them ameliorates pathology (Feb 2017 news; May 2016 conference news). Could shifting the gut microbiota in mice help ameliorate AD-like symptoms?
To find out, some scientists, including Hyunjung Choi, who works in the lab of Inhee Mook-Jung at Seoul National University in South Korea, manipulated the gut microbiome of the ADLPAPT mouse. The researchers created this new model of AD by crossing the 5XFAD strain with JNPL3 tau animals. By about seven months, ADLPAPT mice accumulate both plaques and neurofibrillary tangles, and they lose hippocampal neurons. The latter distinguishes them from most other AD mouse models, which poorly recapitulate the neurodegeneration of AD.
When the ADLPAPT mice were two months old, Choi started transferring fecal microbiota from other mice into their guts. Six ADLPAPT mice received bacteria from age-matched wild-type animals five days a week for 16 weeks, while control ADLPAPT mice got bacteria from other ADLPAPT mice. Four months later, microbiota in the treated ADLPAPT mice resembled that of the wild-type donor. Choi then examined them for changes in Aβ and tau pathology.
Mice receiving wild-type microbiome had half as many Aβ plaques in their frontal cortices and hippocampi as did control ADLPAPT mice. Their hippocampi contained 60 percent less total tau, and their brains had half as much inflammation as measured by staining for Iba1 and GFAP, markers of activated microglia and astrocytes, respectively. These results suggest that the gut microbiome can modulate AD pathology, though the number of animals treated thus far was very small, Choi said.

Cortical boost: Wild-type mice on a control diet (left) have twice as many cortical neurons (red with blue nuclei) as AD mice (middle) at nine months. Give those AD mice a probiotic (right), and their cortical neurons stay at control numbers. [Courtesy of Krista McMurry.]
What about using a probiotic to deliver live “good” bacteria in the gut? Krista McMurry, from the lab of Paola Sacchetti, University of Hartford, Connecticut, supplemented the diets of either wild-type or APPSweTau301 transgenic mice with a probiotic or control inoculum for 12 weeks. The researchers used mice that were five to six months old, the age at which pathology starts. Delivered on a vanilla wafer cookie, the probiotic mixture contained two Lactobacillus strains. The control wafer had saline. Lactobacillus strains commonly appear in probiotic supplements and clinical data suggests they improve symptoms in people with irritable bowel syndrome and reduce inflammation of joints in a mouse model of arthritis (Tiequn et al., 2015; Liu et al., 2016). At the end of the experiment, she examined whether Lactobacillus treatment affected neuron loss, neuroinflammation, or memory.
By the end of the supplementation, APPSweTau301 mice on the control diet had half the number of neurons in their entorhinal cortices as did wild-type mice; however, APPSweTau301 mice on the probiotic maintained neurons at, or even above, control levels (see image above). And while the number of activated astrocytes in the cortices of the control transgenic mice was double that in wild-type mice, probiotic-treated mice had normal astrocyte numbers.
This neuroprotection seemed to carry no functional benefit on the Barnes maze, an illuminated circular platform with 20 holes around the edge, only one of which leads to safety. Mice that ate probiotics made more exploratory head pokes than did wild-type mice, indicating poorer navigational memory. While the probiotic mice explored half as many outlets as did AppSw/Tau controls, they took as long to escape the maze, suggesting they learned no faster.
McMurry said she next will compare activated microglia between samples, analyze neuron and glia levels in the hippocampus, and examine differences in pathological Aβ and tau species.
Similarly, Harpreet Kaur, from the lab of Colin Combs at the University of North Dakota, Grand Forks, found only a hint that a probiotic mix of eight strains of bacteria, including Bifidobacteria and Lactobacilli, improved memory in another mouse model of AD, the NL-G-F mutant APP knock-in. While seven-month-old NL-G-F mice given the probiotic for eight weeks tended to explore novel rather than familiar arms of a cross maze, the difference was not significant. Fewer activated microglia in the temporal cortices of the treated mice suggested the probiotic had some effect on the brain, but levels of soluble and insoluble Aβ40/42 in the temporal cortex were no different than in controls. Steve Estus, University of Kentucky, Lexington, who was not involved in the study, commented that this probiotic might reduce anxiety and related behavior, rather than cognition.
Are there genetic influences on the microbiome? ApoE is a strong risk factor for AD and other diseases, so Estus was curious if there was any cross-talk between its isoforms and the microbiome. Ishita Parikh, then a graduate student in the Estus lab, now a postdoctoral fellow in the lab of Ai-Ling Lin, also at the University of Kentucky, used ApoE targeted replacement (TR) mice, in which one of the human ApoE isoforms occupies the spot in the genome reserved for mouse ApoE in the wild-type. Parikh analyzed fecal DNA from 223 animals, a large number for an academic mouse study. Poop came from four- and six-month-old ApoE2, ApoE3, and ApoE4 TR mice, as well as offspring from TRx5XFAD mouse crosses. The researchers identified different bacteria by DNA sequencing.
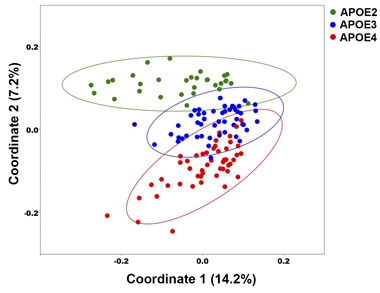
ApoE Shifts Microbiome?
Principal coordinate analysis, based on 14.2 percent and 7.2 percent of the total genetic variation found, displays how similar or dissimilar two microbiomes are. The microbiome of each mouse is represented by a single data point. Similar bacterial profiles appear close together. Bacteria of ApoE2, ApoE3, and ApoE4 mice separate into distinct clusters. [Courtesy of Ishita Parikh.]
Next the researchers used a method called principal coordinate analysis (PCoA) to quantify the similarities and dissimilarities among the different microbiomes. ApoE2, ApoE3, and ApoE4 mice had different bacterial profiles, regardless of whether the mice carried the 5XFAD mutations. Relative to the other genotypes, ApoE2 mice hosted more members of the Ruminococceae family, bacteria that digest starches to short-chain fatty acids. ApoE4 mice had more Lactobacilleae, which have been linked with good gut health. The profile of the ApoE3 mice lay in between. Estus plans to test for ApoE-related microbiome differences in other models of disease.
The upshot of these studies is that the gut microbiome might somehow protect the brain, though the mechanisms are not understood. Lap Ho, from the lab of Giulio Pasinetti at the Icahn School of Medicine at Mount Sinai, New York, examined whether certain bacteria metabolites affected dimerization of synthetic α-synuclein in vitro. Of six compounds Ho tested, one—valeric acid, a short-chain fatty acid produced when bacteria digest dietary fiber—prevented α-synuclein monomers from pairing up and aggregating into fibrils. These scientists previously reported that short-chain fatty acids can inhibit Aβ40 and Aβ42 aggregation (Ho et al., 2018). “This is a side of short-chain fatty acids that I hadn’t considered,” said Estus. “I usually think of them acting in an epigenetic fashion.” Estus wondered what concentration of valeric acid was needed to block α-synuclein dimerization, and how that compared to the concentrations found naturally in the human brain.
These aren’t the only ways intestinal flora could affect the brain. “The human microbiome provides a wealth of highly pro-inflammatory neurotoxins such as lipopolysaccharide, endotoxins, and the enzyme fragilysin,” Walter Lukiw, Louisiana State University, New Orleans, told the audience at SfN. These toxins are normally confined to the human GI tract, he said, but with aging or disease-related breakdown of the GI tract or blood-brain barrier, these neurotoxins may gain access to CNS compartments.
These conference presentations were preliminary and warrant more exploration, researchers agreed. “These early signals are piquing people’s interest,” Petanceska told Alzforum. “We need to be scientifically rigorous, put these early findings to the test, and expand the repertoire of microbiome manipulations to gain more confidence that there is something to them. Equally important is to complement studies in animal models with robust human studies to understand the role of the microbiome in brain aging and AD neurodegeneration.”—Gwyneth Dickey Zakaib
References
News Citations
- Do Microbes in the Gut Trigger Parkinson’s Disease?
- Microbes in the Gut Egg on Aβ Pathology in Mice
- Microbial Hypotheses Intrigue at Zilkha Alzheimer’s Meeting
Research Models Citations
- 5xFAD (B6SJL)
- JNPL3(P301L)
- 3xTg
- APP NL-G-F Knock-in
- APOE2 Targeted Replacement
- APOE3 Targeted Replacement
- APOE4 Targeted Replacement
Paper Citations
- Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of Lactobacillus in treating irritable bowel syndrome: a meta-analysis. Intern Med. 2015;54(3):243-9. PubMed.
- Liu X, Zeng B, Zhang J, Li W, Mou F, Wang H, Zou Q, Zhong B, Wu L, Wei H, Fang Y. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci Rep. 2016 Aug 2;6:30594. PubMed.
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018 Jan;18(1):83-90. Epub 2017 Nov 14 PubMed.
Further Reading
Papers
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 2017 Oct 19;7(1):13537. PubMed.
- Quigley EM. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep. 2017 Oct 17;17(12):94. PubMed.
- Zhao Y, Cong L, Jaber V, Lukiw WJ. Microbiome-Derived Lipopolysaccharide Enriched in the Perinuclear Region of Alzheimer's Disease Brain. Front Immunol. 2017;8:1064. Epub 2017 Sep 4 PubMed.
- Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017 Oct;74(20):3769-3787. Epub 2017 Jun 22 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of California, San Francisco
This article presents important concepts in the discussion of AD pathogenesis. It becomes particularly informative when viewing AD as the result of a progressive energy deficiency syndrome in the CNS.
An essential element is the ability of the gut microbiome to produce materials that serve as an alternate, non-glucose source of metabolizable energy for the CNS. When unabsorbed food components, often polysaccharide in structure, get metabolized by microorganisms of the microbiome, products can include short-chain fatty acids that can then get absorbed into the body. The above involves in vitro research, but it must be considered that after absorption, fatty acids immediately travel to the liver, where they are metabolized into other substances; the precise products depend on the body’s physiological requirements of the moment. Another factor to consider is that before there can be an effect in the central nervous system (CNS), substances must pass through the blood-brain barrier.
This paper mentions valeric acid, a 5-carbon fatty acid. When produced by the microbiome in an intact animal, valeric acid is metabolized by the liver into acetate and propionate, giving rise to both glycogen and ketone bodies. Butyric acid is another short-chain fatty acid produced by organisms in the microbiome; and appears in the blood as β-hydroxybutyrate (BHB), another ketone body, after passage through the liver.
Ketones are an asset to neuroenergetic status, as they cross the blood-brain barrier and serve as an alternate source of metabolizable energy for the CNS. They get produced during starvation or when the body is on a carbohydrate-restricted, “ketogenic” diet. This integrates well with the physiology of the AD process in that ketones represent a potential energy asset at times when available energy in the CNS is becoming rate-limiting for neuronal function.
These concepts help explain why a high-fiber diet, ostensibly providing more substrate for microbiome fermentation and subsequent production of ketones, has been suggested as healthy for the brain. It will be important to identify effective prebiotic-probiotic combinations to optimize this as a CNS energy asset. We need data to assess the effect on memory, cognition, and the etiology of AD.
I am fascinated by the different ApoE isoform effects on the microbiome. One concept serving as evidence to support a neuroenergetic hypothesis is the report that ApoE4 is associated with a decreased baseline ability of glucose to cross the blood-brain barrier, i.e., 29 percent reduction in the mouse. A genetically reduced baseline ability for glucose to cross the blood-brain barrier helps explain the risk of an earlier age of onset in those who carry two copies of ApoE4. It all fits with the neuroenergetic model of AD pathogenesis.
Make a Comment
To make a comment you must login or register.