Right Turn: Aβ Fibril Structure from Alzheimer’s Brain Reveals Surprising Twist
Quick Links
Bucking conventional, i.e., left-leaning, wisdom for amyloid fibrils, Aβ fibrils extracted from the brains of three people with Alzheimer’s disease all twisted to the right. This is apparent in the first-ever structure of Aβ fibrils isolated directly from the AD-ravaged brain, published October 29 in Nature Communications. Researchers led by Marcus Fändrich of Ulm University in Germany reported that the structures differed markedly from those of recombinant fibrils generated in vitro.
- CryoEM yields first structure of Aβ fibrils from human AD brain.
- Fibrils twisted to the right, unlike left-handed structures of recombinant fibrils.
- Fibrils consisted of protofilaments containing dimers of C-shaped Aβ peptides positioned back-to-back.
The researchers detected three varieties of fibril. Each appeared to contain the same protofilament core, which consisted of two C-shaped Aβ peptides tethered back-to-back within the fibril’s center, with their arched N- and C-termini exposed along the surface of the filament.
“This study confirms the importance of structure determination on diseased tissue samples, and provides a first glimpse into the thus-far uncharacterized structures of Aβ filaments in Alzheimer's disease,” commented Sjors Scheres of the MRC Laboratory of Molecular Biology in Cambridge, England, who used cryoEM to uncover the first atomic resolution structure of tau filaments from the AD brain (Jul 2017 news). “In the future, it will be interesting to see whether, in disease, Aβ filaments are as structurally diverse as tau filaments have been shown to be,” Scheres wrote to Alzforum.
Aβ fibrils have been subjected to a plethora of structural probes. Some studies used recombinant protein to grow fibrils in test tubes. Others started with tissue from postmortem brain, but had to amplify the fibrils in vitro to generate enough for analysis (Sep 2013 news; Jan 2017 news). Some scientists trained their efforts on Aβ42; others on Aβ40. Numerous structures have emerged, ranging from helical tubes to U-shaped stacks to tildes, S-bends, and LS curves. Alas, it was never clear how closely they resembled Aβ fibrils in the human brain (Nov 2005 news; Mar 2009 news; Sep 2011 news; May 2015 news; Sep 2017 news).
First author Marius Kollmer and colleagues used cryoEM to resolve the structures of Aβ fibrils isolated from postmortem brain tissue of three people who had died with AD between the ages of 70 and 84. All had numerous neuritic plaques and tangles in the cortex, as well as severe amyloid angiopathy in the leptomeningeal and superficial cortical vessels. The researchers had honed their isolation techniques from patient samples on tissue from people with different types of systemic amyloidosis (Annamalai et al., 2016). In these cases, massive amounts of amyloid crowd several organs in the body, making such fibrils more abundant and easier to purify than those from the brain, Fändrich said.
With isolation techniques in hand, and rapid advances in cryoEM, the scientists decided to tackle Aβ fibrils in the brain, gently isolating and purifying fibrils from the vasculature of the meninges. Fändrich told Alzforum that he opted for the meningeal fibrils because they were more abundant and less prone to contaminants than their parenchymal counterparts. Consistent with previous studies on meningeal Aβ, the purified fibrils comprised primarily Aβ40 peptides, with very little Aβ42 (Charidimou et al., 2017).
An early look at the fibrils by transmission and scanning electron microscopy indicated that, similar to recombinant fibrils seen in the past, these fibrils had a characteristic twisted morphology. However, the similarities ended there. Unlike any in vitro-formed Aβ fibril, these brain-derived filaments twisted to the right.

Turning Right. Side view of right-handed fibril (left). At right, cross-sectional view of a protofilament, consisting of two C-shaped Aβ40 peptides, positioned back-to-back and stacked (left). [Courtesy of Kollmer et al., Nature Communications, 2019.]
Using cryoEM, the scientists spotted three predominant fibril morphologies, which together represented 75 percent of the fibril structures spotted across all of the samples. Each of these three varieties occurred in each of the three donor brains.
The researchers resolved the most common one—morphology I—at the near-atomic resolution of 4.5Å. It consisted of a twisted stack of protofilaments, each of which comprised a dimer of C-shaped Aβ40 peptides. Each Aβ40 peptide had four β sheets, β1-β4. The first three β-sheets formed a U-shaped arch at the N-terminus of the peptide, forming the top of the C. The back of the C did not contain β-sheets, but rather formed extensive contacts with its dimer partner. Finally, the fourth β-sheet helped round out the bottom, C-terminal portion of the C. Polar and hydrophobic residues along the back of the C—most notably, those that made a hump in the center—tethered the two peptides together to make up a single protofilament rung. The N- and C-termini of each Aβ40 peptide were exposed on the fibril surface, while the fibril core contained the connected center portions of the peptides. The protofilament stacks were offset by 2.41Å, giving the fibril its characteristic pseudo 21-screw symmetry.
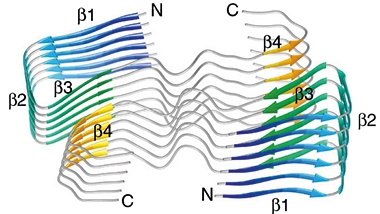
Protofilament Dimers. Six layers of protofilaments, each comprising two C-shaped Aβ40 peptides positioned back-to-back. Each peptide contains four β-sheets, β1 to β4. [Courtesy of Kollmer et al., Nature Communications, 2019.]
The scientists resolved the other two fibril morphologies at 5.65Å and 7.01Å, respectively. These polymorphs appeared to contain the same stacks of paired C-shaped peptides as did morphology I, but differed by the number of protofilament stacks wound into their fibrils. Morphology II had two protofilament stacks wrapped around each other—making for a total of four Aβ40 peptides in each cross-section of the fibril. Morphology III fibrils had three protofilament stacks wound around each other. In rare cases, the researchers observed morphology I and II within the same fibril, where it appeared that one of the protofilament stacks was discontinued. This phenomenon lends further support to the idea that all of the fibril forms shared a common protofilament structure.
“The implication of this work, in my opinion, is a clear demonstration that the Aβ fibrils are polymorphic at the molecular level in patients,” wrote Wei Qiang of Binghamton University in New York. “The origin and biological significance of such polymorphisms are unclear at this point, but worth studying,” he noted.

Morphing Fibrils. A cryo-EM micrograph shows fibril morphologies I and II. In one case (red asterisk), a single fibril contains sections of morphology I (with one protofilament) and II (with two protofilaments). [Courtesy of Kollmer et al., Nature Communications, 2019.]
Qiang pointed out that mutations or modifications to the Aβ sequence could influence the evolution of fibril polymorphisms, though the authors noted that, when projected onto the present structure, most known mutations in the Aβ sequence of APP do not affect fibril structure in an obvious way.
These AD fibrils mark the second reported instance of human pathogenic fibrils with a right-handed twist. The first, also by Fändrich’s group, is the serum amyloid A protein from human systemic AA amyloidosis (Liberta et al., 2019). Considering both structures together, the researchers proposed that their unique peptide folds dictated the right-handedness of the fibrils.
“It is amazing to see the vast structural diversity of Aβ amyloid,” wrote Roland Riek, ETH Zürich, who noted that the unique C-shaped peptide fold, with its large interface between each peptide dimer, is entirely different from any in vitro structure reported. “For me, we are at the beginning of the structural biology of amyloid. There are most likely many, many polymorphs that depend on the aggregation conditions,” Riek wrote.
Just what are the conditions in the brain that allow these unique Aβ fibrils to form? This is a major unanswered question, Fändrich said, and could relate to specific lipids or aminoglycans with which the fibrils interact in the brain. It remains to be seen whether the structure of fibrils extracted from parenchymal plaques—which primarily contain Aβ42—will differ from those Kollmer extracted from the meninges.
Mathias Jucker of the German Center of Neurodegenerative Diseases in Tübingen, a co-author on the paper, noted that the unique structure of fibrils from the human AD brain jibes with the finding that synthetic Aβ makes a poor proteopathic seed compared with brain-derived Aβ assemblies. Conclusions drawn from experiments using synthetic Aβ must be considered carefully, he wrote.—Jessica Shugart
References
News Citations
- Tau Filaments from the Alzheimer’s Brain Revealed at Atomic Resolution
- Does Aβ Come In Strains? Glimpse Into Human Brain Suggests Yes
- Do Palettes of Aβ Fibril Strains Differ Among Alzheimer’s Subtypes?
- See How They Grow: Structure of Amyloid-β Fibrils
- CryoEM Exposes Possible Achilles’ Heel in Aβ1-42 Fibrils
- Will The Toxic Aβ Structure Please Stand Up?
- Danger, S-Bends! New Structure for Aβ42 Fibrils Comes into View
- Amyloid-β Fibril Structure Bares All
Paper Citations
- Annamalai K, Gührs KH, Koehler R, Schmidt M, Michel H, Loos C, Gaffney PM, Sigurdson CJ, Hegenbart U, Schönland S, Fändrich M. Polymorphism of Amyloid Fibrils In Vivo. Angew Chem Int Ed Engl. 2016 Apr 4;55(15):4822-5. Epub 2016 Mar 8 PubMed.
- Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017 Jul 1;140(7):1829-1850. PubMed.
- Liberta F, Loerch S, Rennegarbe M, Schierhorn A, Westermark P, Westermark GT, Hazenberg BP, Grigorieff N, Fändrich M, Schmidt M. Cryo-EM fibril structures from systemic AA amyloidosis reveal the species complementarity of pathological amyloids. Nat Commun. 2019 Mar 7;10(1):1104. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Kollmer M, Close W, Funk L, Rasmussen J, Bsoul A, Schierhorn A, Schmidt M, Sigurdson CJ, Jucker M, Fändrich M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer's brain tissue. Nat Commun. 2019 Oct 29;10(1):4760. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
MRC Laboratory of Molecular Biology
This paper was a lot of fun to read. The authors describe the structure of Aβ amyloid filaments that were extracted from meningeal Alzheimer’s brain tissue. As we previously also observed for tau filaments, the Aβ filaments form different structures in the brain from those obtained through in vitro aggregation methods.
Interestingly, as some of these authors also described for serum amyloid A protein from human systemic AA amyloidosis, the Aβ filaments are right-handed, despite amyloid filaments having a natural tendency to be left-handed due to the natural twist of β-strands.
The authors also found polymorphism in the Aβ filaments, which again is reminiscent of the polymorphism found in tau, as the same protofilaments can pack against each other in different ways. For Aβ, filaments with one, two, or three protofilaments were observed. Molecular details of the structure remain at large due to the somewhat limited resolution, which can be explained by the limited mass of the filament per unit length.
This study confirms the importance of structure determination on diseased tissue samples, and provides a first glimpse into the thus-far uncharacterised structures of Aβ filaments in Alzheimer's disease. In the future, it will be interesting to see whether, in disease, Aβ filaments are as structurally diverse as tau filaments have been shown to be.
ETH Zurich
This work by Jucker and Fändrich is a very important step forward in the structural biology of Aβ, since the amyloid has been purified from human AD material. This is the first structure of its kind (a solid state NMR structure of Aβ(1-40) has been determined before from seeded material using purified brain homogenate by the Tycko group at NIH).
Highly important to mention is the purification procedure. It is based on an old procedure from Pras et al., 1968, which is apparently based on another purification protocol from Cohen's group. Unfortunately, the details of the present purifications are not given, but the purification is based on water solubility, which is indeed gentle.
The structure is entirely different from previous, in vitro-derived ones. At the mesosopic scale, it is right-hand twisted and not left-hand twisted, as most of the other fibrils. At the atomic structure, an entire different fold is present, which also includes different segments in the sequence (starting from residue 2, ending already at residue 37). It has a rather large interface between two molecules, etc. It is amazing to see the vast structural diversity of an Aβ amyloid.
For me, we are at the beginning of the structural biology of amyloid. There are most likely many, many polymorphs that depend on the aggregation conditions. For example, in the case of α-synuclein, there are two very distinct polymorphs likely to be attributable to whether there is phosphate in the buffer or not. Furthermore, based on thermodynamics, all the polymorphs should be present in a sample, but just at different concentrations. Depending on the environment, one polymorph is more present than others because it replicates faster.
In the AD-derived amyloid presented in this study, all three patients show the same set of polymorphs, indicating a common origin. On the other hand, it could be also that the purification protocol did select this specific polymorph.
References:
Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin EC. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924-33. PubMed.
Binghamton University, SUNY
This structure is in fact quite different from the Aβ40 fibrils grown in vitro (from incubation of synthetic or expressed Aβ40 in aqueous), and also different from a previously published structure of ex vivo fibrils by Tycko et al. The quaternary interface involves two extended β-strands (K16-E22 and A30-M35). These two strands are usually folded to form an intramolecular interface in other structures. Also, the N-terminus looks more ordered with a third extended β-sheet.
The implication of this work, in my opinion, is a clear demonstration that the Aβ fibrils are polymorphic at the molecular level in patients. The origin and the biological significance of such polymorphisms are unclear at this point but worth studying. Also, as pointed by the authors in the discussion, the presence of mutations and modifications in Aβ sequences will have non-negligible influences on the molecular structures of resulting fibrils and may also contribute to the time evolution of polymorphisms.
Make a Comment
To make a comment you must login or register.