CryoEM Exposes Possible Achilles’ Heel in Aβ1-42 Fibrils
Quick Links
The Aβ42 peptide may self-aggregate to form fibers with a structure profoundly different from those made of its less pathogenic cousin Aβ40. So suggests a cryoelectron microscopy (cryoEM) study—the first reported for Aβ1-42—in this week’s PNAS Early Edition. In contrast to structural studies of Aβ1-40 that have predicted a tight arrangement of protofilaments in mature fibers, the new model describes Aβ1-42 fibers as pairs of loosely connected protofilaments snaked around a hollow core. “There might be a weak link in this seemingly impenetrable molecule,” said neurologist Jin-Moo Lee of Washington University School of Medicine, St. Louis, Missouri, who led the new research. If fibrillar Aβ42 “is in fact a toxic species, then this is a potential site where we might disrupt the fibril,” Lee said. The authors and other scientists caution, though, that differences in growth conditions for the various Aβ species make it hard to directly compare structural studies of Aβ1-40 and Aβ1-42. The relevance of synthetic Aβ fibril structures—the kind used here—to Alzheimer disease also remains to be seen.
Because Aβ fibers are such monstrous assemblies that crystallize poorly, scientists have turned to cryoEM to probe their fine structural features. CryoEM allows analysis of large molecules in their native state, albeit at lower resolution than other methods such as X-ray crystallography and nuclear magnetic resonance (NMR). Most structural studies of fibrillar Aβ have focused on those made of Aβ1-40 peptides. Though less abundant than Aβ1-42 in amyloid plaques, Aβ1-40 peptides can more easily assemble into fibrils in the lab. According to a recent cryoEM study by Niko Grigorieff of Brandeis University, Waltham, Massachusetts, and Marcus Fändrich of Max-Planck Research Unit for Enzymology of Protein Folding in Halle, Germany, Aβ1-40 peptides assemble into protofilaments that pack tightly in pairs to form mature amyloid fibrils (Sachse et al., 2008).
Several years ago, a team led by Roland Riek at the Salk Institute for Biological Studies in La Jolla, California, managed to sneak a peek at the more unruly Aβ42 peptide. Using a combination of NMR, mutagenesis, and cryoEM, the researchers revealed a critical Aβ42 feature—a hairpin turn connecting the two β strands of residues 18-42 (see ARF related news story and Lührs et al., 2005). “What we’re contributing here is a more global model,” Lee said. “We looked at the fibril itself, not the stacks of Aβ within the fibril.”
Teaming up with biochemist Carl Frieden, also at Washington University, and cryoEM expert Wah Chiu at Baylor College of Medicine in Houston, Texas, Lee, first author Rui Zhang, and colleagues determined the three-dimensional structure of fibrils made from soluble human synthetic Aβ1-42. Each consisted of two intertwined protofilaments, as was seen for Aβ1-40 fibrils. Individual Aβ1-42 fibrils had quite a bit of structural variability—some were tightly twisted, whereas others adopted a looser configuration. The researchers categorized the fibrils into six groups according to helical periodicity, or pitch length, ranging from 400 to 650 angstroms. They used hundreds of overlapping images in each group to construct density maps, then averaged those to create the final structure.
“What's surprising is that this model of the Aβ1-42 fibril looks very different from the Aβ1-40 fibril,” Lee told ARF. The Aβ1-40 models “don't have this hollow core that we see.” The new model suggests that the two Aβ1-42 protofilaments forming the long, tube-like structure are themselves loosely connected. “We think this interaction is a little slippery,” Lee said. The protofilaments “slide a little so some fibrils can be tighter than others.” Furthermore, he said the NMR hairpin model by Riek and colleagues fits nicely within the spatial parameters of the cryoEM map, providing a measure of confidence in the new reconstruction.
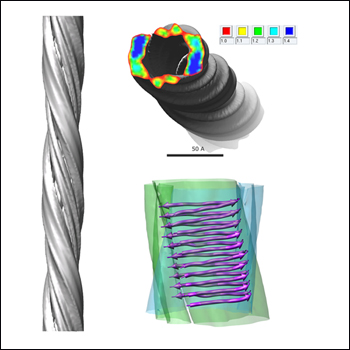
Not a Rope, But a Tube
According to a new cryoelectron microscopy model, Aβ1-42 fibrils adopt a long, helical structure (left) with a hollow core delineated by two loosely connected protofilaments (top right). The fibril structure (bottom right) accommodates a stack of 10 Aβ1-42 molecules with the hairpin predicted by earlier NMR studies. Image credit: Rui Zhang and Wah Chiu
However, some scientists noted that the fibril growth conditions in the new study could make the findings hard to interpret. Lee’s team synthesized the Aβ1-42 fibrils by incubating pure peptide at 37 degrees C for four weeks at pH 2.0. “Incubation at strongly acidic conditions and for a prolonged time is generally known to lead to peptide fragmentation or other covalent modifications,” Grigorieff and Fändrich wrote in an e-mail to ARF. “Therefore, it is possible that the analyzed fibrils differ quite substantially from the ones that are present in Alzheimer patients and that are formed, of course, under physiologically relevant pH conditions.” (See full comment below.) By comparison, the Aβ1-40 fibrils used in previous (Sachse et al., 2008) and more recent structural studies by these researchers (Meinhardt et al., 2009) and others (Paravastu et al., 2008) were produced by incubating peptide at pH 7-9 for several days at room temperature or 4 degrees C.
Lee agrees that growth conditions likely influence fibril morphology. However, he noted that the new model “is not that different from some other (non-Aβ) forms of amyloid. There's some kind of theme underlying some of this,” he said. “SH3 peptide also has two forms of protofilaments surrounding a hollow core (Jimenez et al., 1999).”
Whether the fibril structures predicted in the new study are relevant to AD remains unclear at this point, suggested Rob Tycko of the National Institutes of Health, Bethesda, Maryland. In an e-mail to ARF, he wrote, “…the Aβ1-40 peptide (and possibly also the Aβ1-42 peptide) can probably form five or six different fibril structures. It will be interesting to identify the structure or structures that develop in the human brain.” (See full comment below.)
It is also intriguing to speculate as to whether the Aβ morphological variations might correlate with pathogenicity, noted Huilin Li of Brookhaven National Laboratory, Upton, New York, who uses cryoEM to probe the inner workings of γ-secretase (see ARF related conference story). “As Aβ fibers are highly heterogeneous and polymorphic, it will be interesting to find out whether the structural differences observed in these studies merely reflect the peptide constituents (i.e., Aβ40 versus Aβ42) of the particular species of fibers selected for 3D reconstruction, or whether the structural differences represent a true defining feature of two functionally different peptides (i.e., Aβ40 is significantly less toxic than Aβ42),” wrote Li in an e-mail to ARF. (See full comment below.)
Such considerations could turn out to be moot if Aβ fibrils are not in fact the primary toxic species in AD. This idea has gained traction in recent years with accumulating evidence that disease progression may track more closely with soluble Aβ oligomers than with insoluble deposits. With this in mind, Edward Olejniczak of Abbott Laboratories in Abbott Park, Illinois, and other researchers there and at Abbott GmbH in Ludwigshafen, Germany, have used solution NMR to determine the structure of oligomers made from synthetic Aβ and fatty acids. These soluble “globulomers” appear to be relevant in vivo (see ARF related news story and Barghorn et al., 2005). According to the authors, company policy precluded a phone interview about this published work, but Olejniczak told ARF via e-mail that ongoing preclinical studies with these globulomers will guide future immunotherapy trials. Based on the structural NMR data, Olejniczak, first author Liping Yu, and colleagues have engineered a disulfide bond into their Aβ globulomer to create a stable new antigen that can be therapeutically targeted independent of fibrillar forms. The work appeared online 13 February in the journal Biochemistry (Yu et al., 2009).—Esther Landhuis
References
News Citations
- See How They Grow: Structure of Amyloid-β Fibrils
- Eibsee: Channel Vanishes in Sharper Image
- SfN: Amyloid Oligomers—Not So Elusive, After All? Part 1
Paper Citations
- Sachse C, Fändrich M, Grigorieff N. Paired beta-sheet structure of an Abeta(1-40) amyloid fibril revealed by electron microscopy. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7462-6. PubMed.
- Lührs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Döbeli H, Schubert D, Riek R. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc Natl Acad Sci U S A. 2005 Nov 29;102(48):17342-7. PubMed.
- Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Abeta(1-40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol. 2009 Feb 27;386(3):869-77. PubMed.
- Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer's beta-amyloid fibrils. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18349-54. PubMed.
- Jiménez JL, Guijarro JI, Orlova E, Zurdo J, Dobson CM, Sunde M, Saibil HR. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999 Feb 15;18(4):815-21. PubMed.
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005 Nov;95(3):834-47. PubMed.
- Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET. Structural characterization of a soluble amyloid beta-peptide oligomer. Biochemistry. 2009 Mar 10;48(9):1870-7. PubMed.
Further Reading
Papers
- Lührs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Döbeli H, Schubert D, Riek R. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc Natl Acad Sci U S A. 2005 Nov 29;102(48):17342-7. PubMed.
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005 Nov;95(3):834-47. PubMed.
- Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Abeta(1-40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol. 2009 Feb 27;386(3):869-77. PubMed.
- Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer's beta-amyloid fibrils. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18349-54. PubMed.
- Sachse C, Fändrich M, Grigorieff N. Paired beta-sheet structure of an Abeta(1-40) amyloid fibril revealed by electron microscopy. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7462-6. PubMed.
Primary Papers
- Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET. Structural characterization of a soluble amyloid beta-peptide oligomer. Biochemistry. 2009 Mar 10;48(9):1870-7. PubMed.
- Zhang R, Hu X, Khant H, Ludtke SJ, Chiu W, Schmid MF, Frieden C, Lee JM. Interprotofilament interactions between Alzheimer's Abeta1-42 peptides in amyloid fibrils revealed by cryoEM. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4653-8. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
NIDDK, NIH
1. Before comparing structural studies of Aβ fibrils from different laboratories, it is crucially important to compare the conditions under which the fibrils were grown, as our own solid-state NMR and electron microscopy studies have shown that Aβ fibril structures depend strongly on growth conditions. In the recent cryoEM studies by Zhang et al., the fibrils were grown at 37 C in 10 mM HCl. In our solid-state NMR studies, fibrils were grown at room temperature in pH 7.4 buffer.
2. The Aβ1-40 and Aβ1-42 peptides apparently adopt quite similar molecular conformations in amyloid fibrils, and both form parallel β-sheets, based on solid-state NMR, H/D exchange, and other data. But other aspects of the fibril structures may be somewhat different. Structures of Aβ1-42 fibrils have not yet been characterized completely by solid-state NMR.
3. The most surprising aspect of the cryoEM reconstruction reported by Zhang et al. is the central pore in the Aβ1-42 fibril structure. Structural models for Aβ1-40 fibrils based on solid-state NMR and electron microscopy (especially scanning transmission electron microscopy) do not contain such a large pore. Solid-state NMR data for Aβ1-40 fibrils indicate intermolecular contacts that would be inconsistent with the cryoEM results of Zhang et al. But again, the experiments of Zhang et al. were performed on Aβ1-42, rather than Aβ1-40, and the pH and temperature during fibril growth were quite different.
4. Finally, the Aβ1-40 peptide (and possibly also the Aβ1-42 peptide) can probably form five or six different fibril structures. It will be interesting to identify the structure or structures that develop in the human brain. This is one of the goals of our own current work.
Max-Planck Research Unit for Enzymology of Protein Folding
Zhang at al. report a three-dimensional reconstruction of an Aβ1-42 amyloid fibril based on cryoelectron microscopy data. The obtained structure varies very significantly from the fibril structure that our groups have published for Aβ1-40 peptide. This does not only hold for the Aβ1-40 structure quoted by the authors (Sachse et al., 2006; Sachse et al., 2008). It is also true for a very recently published analysis of the structure of 12 Aβ1-40 amyloid fibrils (Meinhardt et al., 2009) None of them are similar to the Aβ(1-42) fibril structure reported here.
The now published Aβ1-42 fibrils were obtained by in-vitro incubation of pure peptide at pH 2.0 for four weeks. Incubation at strongly acidic conditions and for a prolonged time is generally known to lead to peptide fragmentation or other covalent modifications. Furthermore, different pH values can lead to dramatically different fibril structures. Therefore, it is possible that the analyzed fibrils differ quite substantially from the ones that are present in Alzheimer patients and that are formed, of course, under physiologically relevant pH conditions.
It would be helpful if the manuscript provided more of the technical information that a reader would like to know for judging the reliability of this new Aβ1-42 structure and whether it truly reflects the structure of the analyzed fibrils. For example, comparisons between the raw images obtained in the electron microscope and projections of the structure are not included. No statistical analysis of the different observed fibril symmetries is shown. No mass-per-length measurements were carried out to support the interpretation of the structure with two peptides in cross-section. It is not clear to us why the published structure does not show more structural detail despite its resolution of 10 angstroms. In the light of these concerns, the presented structural model remains speculative at this point, and its relevance for Alzheimer disease remains to be further clarified.
References:
Sachse C, Xu C, Wieligmann K, Diekmann S, Grigorieff N, Fändrich M. Quaternary structure of a mature amyloid fibril from Alzheimer's Abeta(1-40) peptide. J Mol Biol. 2006 Sep 15;362(2):347-54. PubMed.
Sachse C, Fändrich M, Grigorieff N. Paired beta-sheet structure of an Abeta(1-40) amyloid fibril revealed by electron microscopy. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7462-6. PubMed.
Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Abeta(1-40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol. 2009 Feb 27;386(3):869-77. PubMed.
Brookhaven National Laboratory
Aβ40 and Aβ42 are 40- and 42-residue peptides produced by the sequential cleavage of amyloid precursor protein by β-secretase and γ-secretase. The peptides have a strong tendency to self-aggregate, initially into soluble oligomers, and eventually into insoluble fibrils and large neuronal deposits. Although the soluble oligomers are considered the major culprit of neuronal toxicity, there is nevertheless strong interest in the structure of the Aβ fibrils. Aβ fibrils have been a longstanding subject of various biophysical studies, including cryoEM. Nevertheless, the cryoEM structure of Aβ42 fiber at 10-angstrom resolution as reported by Lee and colleagues represents a significant step forward in our pursuit of the structural basis of Aβ peptide fibrillization. The new structure reveals the expected two protofilaments twisted along the fiber axis. The novelty of the new structure is that the β-sheets are arranged at the periphery surrounding a hollow core, thus forming a long tube-like structure. This architecture is drastically different from the fiber structure formed by Aβ40 peptide, also determined by cryoEM, in Niko Grigorieff’s lab at Brandeis University, Waltham, Massachusetts, and reported previously (Meinhardt et al., 2009; Sachse et al., 2008). In the Aβ40 fiber, the β-sheets are arranged radially, twisting along the helical axis to form the long fiber with a solid core. As Aβ fibers are highly heterogeneous and polymorphic, it will be interesting to find out whether the structural differences observed in these studies merely reflects the peptide constituents (i.e., Aβ40 versus Aβ42) of the particular species of fibers selected for 3D reconstruction, or whether the structural differences represent a true defining feature of two functionally different peptides (i.e., Aβ40 is significantly less toxic than Aβ42).
The new cryoEM map by Zhang et al. fits the cryoEM micrograph well and appears solid. Furthermore, the structure model derived from the cryoEM map is supported by their extensive proteolysis data. Nevertheless, the interpretative model shall be taken with a grain of salt. Since the accurate mass per unit length of Aβ42 fiber is not known in this case, the display threshold for surface-rendering of the cryoEM map has to be somewhat artificial. The choice of threshold would thus have implications in building the structural model. I also want to point out that the proteolysis data, although supportive of their model, is not in conflict with a previous model that involves inter-β-sheets interaction (Sato et al., 2006). In summary, my impression is that there is a need for understanding the structural basis of Aβ peptide fibrillization. The current work might not be the final elucidation of such mechanism, but is a significant step forward in the long quest.
References:
Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Abeta(1-40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol. 2009 Feb 27;386(3):869-77. PubMed.
Sato T, Kienlen-Campard P, Ahmed M, Liu W, Li H, Elliott JI, Aimoto S, Constantinescu SN, Octave JN, Smith SO. Inhibitors of amyloid toxicity based on beta-sheet packing of Abeta40 and Abeta42. Biochemistry. 2006 May 2;45(17):5503-16. PubMed.
�
CryoEM-determined structures of Alzheimer’s peptide Aβ1-42 reveal significant differences between the fibrils of this peptide and the other most-studied Alzheimer’s peptide, Aβ1-40. Thus, they extend the known differences in kinetic, thermodynamic, and dynamic properties of these two peptides observed in solution to the supramolecular architecture of fibrils formed by them.
One of the significant points of this study is that fibrils formed by Aβ1-42 have a hollow core in contrast to those formed by Aβ1-40. At a cross-sectional plane, each protofilament accommodates a single molecule of Aβ1-42 in a hairpin-like conformation while two Aβ1-40 peptides are present in extended conformation in their respective fibrils. Structures of both fibrils were determined to the same resolution (~10 angstrom vs. ~8 angstrom); therefore, the differences can’t be attributed to the differences in experimental data collection.
However, fibril morphology is highly dependent on growth conditions. Under a variety of growth conditions, a different conformation from an ensemble of conformations may prevail under a given set of conditions for each peptide. Nevertheless, data shown in this work are consistent with the differences observed between the two peptides in solution studies. They remind us that we still don’t know the nature of molecular interactions that affect these two similar peptides such that they can behave so differently in solution leading to significantly different consequences.
One of the common points between the EM structures of both peptides is that both structures suggest protofilaments are joined through the flexible N-terminal residues of both peptides, which also agree with the solution studies. Thus, it is very likely that dynamic properties of these peptides (i.e., switching between various conformations and their thermodynamic consequences) play a significant role in determining how the individual peptides form the initial complex and extend it to a protofilament and fibril level. This would allow small differences to be amplified, yielding significant kinetic and structural differences in fibrils of the same peptide or between the fibrils of the two peptides.
Make a Comment
To make a comment you must login or register.