Young ApoE4 Carriers Have Reversed AD Proteomic Signature
Quick Links
APOE4 is the strongest genetic risk factor for sporadic Alzheimer’s disease, but at what age does the risk start to play out? A new study hints that it might be as young as one's 30s. In the November 10 Science Advances, researchers led by Madhav Thambisetty, National Institute on Aging, Baltimore, reported that 24 proteins upregulated in the brains of young adult APOE4 carriers are part of an AD proteomic signature. Curiously, these 24 proteins were downregulated in AD, while one other protein that was downregulated in young APOE4 carrier brains was upregulated in AD. The authors don’t understand this dichotomy. Still, levels of some of these proteins tracked with plaques, tangles, or cognitive decline.
- The same two dozen proteins are upregulated in young adult APOE4 carriers, downregulated in AD.
- Dasatinib, an inhibitor of two of them, lowered tau in cell cultures.
- The drug, a senolytic, is in Phase 2 for AD.
Some of these proteins also are targets of approved drugs that have drawn interest for repurposing in AD. Two, the kinases Yes1 and Fyn1, are blocked by the anti-cancer drug dasatinib, a senolytic that is already being tested in Alzheimer's. At the Clinical Trials on Alzheimer's Disease conference held online and in Boston, November 9-12, Miranda Orr of Wake Forest School of Medicine, Winston-Salem, North Carolina, outlined the design of a Phase 2 trial starting this month. Called, rather graphically, SToMP-AD, aka Senolytic Therapy to Modulate the Progression of Alzheimer's Disease, it will test a combination of dasatinib and the anti-inflammatory compound quercetin in people with early AD. An ongoing, open-label study in AD suggests the combo is safe, Orr said. The work comes amid twin efforts to revive drug repurposing research in AD and to develop senolytic therapies both old and new.
Proteomic Signature
Orr praised Thambisetty’s approach, which compared proteomic signatures from different regions of the AD brain with those from young APOE4 carriers. “The use of tissue from multiple ages is important to appropriately interpret results and guide the development and use of therapeutic interventions,” she wrote (full comment below).
First author Jackson Roberts collected middle frontal and inferior temporal gyrus tissue from 62 people who had had AD and from 41 healthy controls in the Baltimore Longitudinal Study of Aging (BLSA) or Chicago's Religious Orders Study (ROS). Their average age at death was 89. Quantifying 1,300 proteins within the samples, the scientists identified 120 as either up- or downregulated in AD relative to controls.
Next, the scientists analyzed brain tissue from the Young APOE Postmortem Study; average age at death, 39. YAPS collects tissue from people who died of trauma and had forensic autopsies at the Baltimore coroner’s office. Co-author Juan Troncoso and colleagues at Johns Hopkins obtained samples from 18 APOE4 carriers and 17 age-matched noncarriers for APOE genotyping and storage.
Among the 120 proteins that together constituted the BLSA/ROS AD signature, 25 were expressed differently between the young E4 carriers and the noncarriers (see image below). “It is striking to see protein differences in E4 carriers decades before symptom onset,” said Giacomo Koch, Fondazione Santa Lucia, Rome, who participated in a panel discussion on drug repurposing at CTAD. The scientists don’t know if these carriers would have developed AD had they lived.
What do these 25 proteins do? Gene set enrichment analysis indicated some shared roles, including tyrosine kinase signaling. “Some signaling pathways, including inflammation, cell cycle arrest, and the autophagic-lysosomal network, suggest a role for senescence in the E4 and AD changes,” Orr told Alzforum.
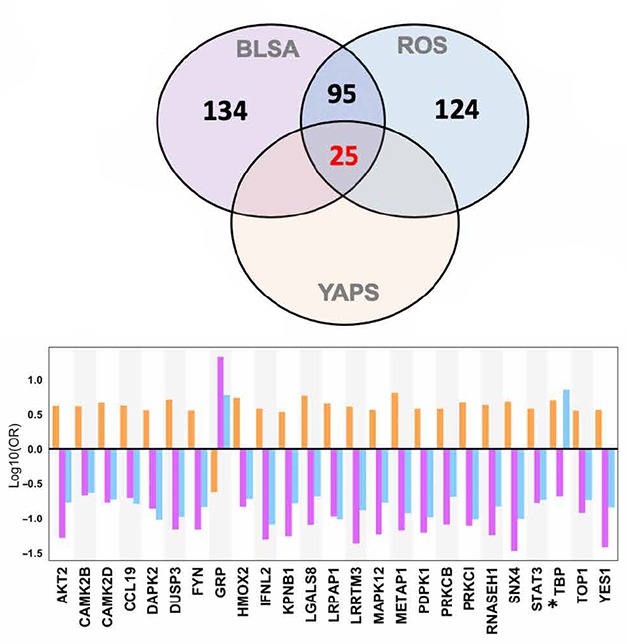
Compare and Contrast. In AD brain tissue, 120 proteins were differentially expressed (purple and blue circles). Twenty-five of those distinguished young APOE4 carriers from noncarriers (orange circle). For the most part, expression change in E4 carriers (orange bars) was opposite that in AD (purple, blue bars). [Courtesy of Roberts et al., Science Advances, 2021.]
In the AD cases, 21 of the 25 proteins tracked with amyloid plaque deposition; 15 tracked with tau tangles according to Braak stage. Three of the 25 associated with slower cognitive decline via MMSE, and another three with faster decline.
Oddly, levels of all but one of the 25 proteins were more highly expressed in the young E4 carriers, but downregulated in the AD cases. Thambisetty thinks the changes in the young carriers may be driving the pathogenesis but in later years, as protein upregulation cannot be sustained over time, expression wanes. “Our results provide a window into very early biological perturbations occurring during the long preclinical phase of AD that may present novel therapeutic targets for disease modification," the authors wrote.
Of the 25 proteins, six turned out to be targets of existing drugs. They are tyrosine kinases Fyn1 and Yes1; the secreted lectin Lgals8; protein phosphatase Dusp3; the cytokine transducer Stat3; and topoisomerase 1. The scientists chose to study the Fyn1/Yes1 inhibitor dasatinib, which is FDA-approved to treat chronic myeloid leukemia (Lindauer et al., 2010). When treated with dasatinib, SH-SY5Y neuroblastoma cultures overexpressing a human tau fragment with the V337M and R406W mutations made less phospho-tau 231 and total tau (see image below).
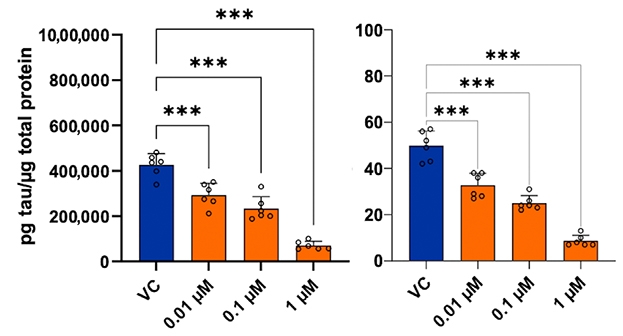
Tempering Tau? In cell lines overexpressing human tau with tauopathy mutations, dasatinib reduced total tau (left) and phospho-tau 231 (right). [Courtesy of Roberts et al., Science Advances, 2021.]
Koch questioned whether hand-picking targets based on drug availability will work. “It is a bit of a long shot,” he said. “Just because drugs are available, especially if they are cancer drugs, does not mean they will be useful in AD if they are too toxic to take long-term.”
Another question is whether the proteins downregulated in AD would benefit from further quashing, as happens in kinase inhibition with dasatinib. Mitzi Gonzales, University of Texas Health Science Center, San Antonio, called Thambisetty's findings encouraging but agreed that the opposite direction of expression between young E4 carriers and old people with AD highlights the disease's complexity. “We should be careful about timing potential therapeutics, because a drug that works in the preclinical stage may not work for clinical disease,” she told Alzforum.
For their part, Thambisetty's team will study dasatinib in mouse models and analyze existing clinical data for correlation between dasatinib use and cognitive problems via the Drug Repurposing for Effective Alzheimer’s Medicines study (Desai et al., 2020).
On the other hand, a Phase 2 senolytic trial run by Gonzales and Orr is starting to test dasatinib together with the flavonoid quercetin directly in AD (Gonzales et al., 2021).
“Identifying dasatinib at the convergence of two independent drug screens, one focused on AD pathogenesis and the other on senescent cells, is an exciting finding that lends support to the overall strategy,” Orr wrote.
SToMP to the Clinic
The combination of dasatinib and quercetin triggers cell death in senescent cells, i.e., cells that have outlived their usefulness but resist apoptosis (Gonzales et al., 2021).
Senescent cells are thought to exacerbate aging phenotypes and age-related diseases, including AD (Oct 2021 news; Jun 2021 news; Jul 2021 news).
In mouse models of tauopathy or amyloidosis, the drug combo has been reported to clear senescent brain cells, reduce tangle or plaque load, decrease neuroinflammation, slow brain atrophy, and improve memory behaviors (Sep 2018 news; Apr 2019 news).
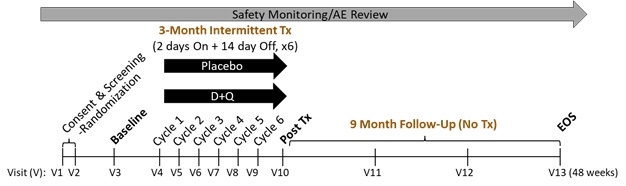
SToMPing Along. A trial will give short bursts of low-dose dasatinib and quercetin to people with MCI and early AD, then follow up nine months later. [Courtesy of Miranda Orr, Wake Forest School of Medicine.]
SToMP-AD will recruit 48 people over 65 with MCI or early AD who test positive for tau by PET or CSF. They will take the drugs or placebo for two days, wait two weeks, then repeat, for a total of six rounds (see image above). Senolytic drugs are given intermittently because cells take time to become senescent. The dose is almost 10 times less than what is given for cancer.
After three months of treatment, the researchers will assess participants’ cognition and measure senescence markers in their blood. Measurements will be repeated at three, six, and nine months post-treatment, with a tau PET scan at the final visit. The primary outcome is safety, with secondaries of change in CDR-SB and ADAS-Cog, tau PET, and senescence blood markers. Recruitment is starting this month.
This regimen of low-dose bursts could allow patients to skirt the known toxicity of the drug. “So far, this low-dose dasatinib plus quercetin has been safe in three pilot studies, including our open-label pilot in older adults with AD,” Orr said. This pilot also tests if dasatinib/quercetin enters the brain. Five older adults with early AD took both drugs in the same intermittent cycles for three months, then had levels of each drug, total tau, Aβ42, and senescence markers measured in CSF and blood. MRIs were done at baseline and three months; CDR and MoCA at baseline, three, and six months.
At CTAD, Orr said that the final six-month follow-ups are almost complete, and that the regimen was safe. Gonzales, who leads this trial, told Alzforum that no one discontinued treatment and there were no serious adverse events. All other data analysis is ongoing. Gonzales plans to present top-line results at ADPD in March.
The other two pilots studied the drug combo in idiopathic pulmonary fibrosis and diabetic kidney disease (Justice et al., 2019; Hickson et al., 2019).
A separate open-label pilot, of the same intermittent dosing of dasatinib/quercetin in 20 people with MCI or early AD, is slated to read out in June 2023. Called ALSENLITE, its only outcome is safety. “So far [dasatinib and quercetin] seem safe in SToMP-AD and in ongoing studies for other indications, but it is too early to be assured,” study PI James Kirkland of Mayo Clinic wrote to Alzforum
Beyond dasatinib, research on senolytic compounds is growing rapidly. Kirkland is a co-author on an upcoming study led by Yu Sun, Chinese Academy of Sciences, Shanghai, that identified the flavonoid procyanidin C1 from grape seed extract as senolytic (Xu et al., 2021). Much of the paper focuses on cancer, but it also reports that intermittent administration of PCC1 to old mice reduced their burden of senescent cells, strengthened their physical activity, and lengthened their lifespans.—Chelsea Weidman Burke
References
Therapeutics Citations
News Citations
- After Eating Tangle-Tainted Neurons, Microglia Spew Tau, Lose Appetite
- DAMned to Death? Microglia May Proliferate to Senescence
- Astrocytes Are Just Dying to Spread Tau
- Are Tauopathies Caused by Neuronal and Glial Senescence?
- Plaques Age Glial Precursors, Stoking Inflammation
Paper Citations
- Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2010;184:83-102. PubMed.
- Desai RJ, Varma VR, Gerhard T, Segal J, Mahesri M, Chin K, Nonnenmacher E, Gabbeta A, Mammen AM, Varma S, Horton DB, Kim SC, Schneeweiss S, Thambisetty M. Targeting abnormal metabolism in Alzheimer's disease: The Drug Repurposing for Effective Alzheimer's Medicines (DREAM) study. Alzheimers Dement (N Y). 2020;6(1):e12095. Epub 2020 Nov 26 PubMed.
- Gonzales MM, Garbarino VR, Marques Zilli E, Petersen RC, Kirkland JL, Tchkonia T, Musi N, Seshadri S, Craft S, Orr ME. Senolytic Therapy to Modulate the Progression of Alzheimer's Disease (SToMP-AD): A Pilot Clinical Trial. J Prev Alzheimers Dis. 2022;9(1):22-29. PubMed.
- Gonzales MM, Krishnamurthy S, Garbarino V, Daeihagh AS, Gillispie GJ, Deep G, Craft S, Orr ME. A geroscience motivated approach to treat Alzheimer's disease: Senolytics move to clinical trials. Mech Ageing Dev. 2021 Dec;200:111589. Epub 2021 Oct 21 PubMed.
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019 Feb;40:554-563. Epub 2019 Jan 5 PubMed.
- Hickson LJ, Langhi Prata LG, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019 Sep;47:446-456. Epub 2019 Sep 18 PubMed.
- Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, He R, Zhang X, Ju Z, Campisi J, Kirkland JL, Sun Y. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. 2021 Dec 6; PubMed.
External Citations
Further Reading
Papers
- Maudsley S, Devanarayan V, Martin B, Geerts H, Brain Health Modeling Initiative (BHMI). Intelligent and effective informatic deconvolution of "Big Data" and its future impact on the quantitative nature of neurodegenerative disease therapy. Alzheimers Dement. 2018 Jul;14(7):961-975. Epub 2018 Mar 15 PubMed.
Primary Papers
- Roberts JA, Varma VR, An Y, Varma S, Candia J, Fantoni G, Tiwari V, Anerillas C, Williamson A, Saito A, Loeffler T, Schilcher I, Moaddel R, Khadeer M, Lovett J, Tanaka T, Pletnikova O, Troncoso JC, Bennett DA, Albert MS, Yu K, Niu M, Haroutunian V, Zhang B, Peng J, Croteau DL, Resnick SM, Gorospe M, Bohr VA, Ferrucci L, Thambisetty M. A brain proteomic signature of incipient Alzheimer's disease in young APOE ε4 carriers identifies novel drug targets. Sci Adv. 2021 Nov 12;7(46):eabi8178. Epub 2021 Nov 10 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UT Health San Antonio/South Texas VA
The study by Roberts and colleagues was designed to identify differential protein expression in AD that may be relevant to preclinical stages or risk for developing AD later in life. The experimental design allowed the researchers to identify drug targets that may be relevant in early disease. This is important because it may allow for treatment prior to neurodegeneration and cognitive impairment.
The directionality differences in protein expression in YAPS versus ROS and BLSA have important implications for drug discovery and development. Future experiments are needed to determine if/how to appropriately modulate these pathways during different disease stages. A sub-analysis focused on APOe4 carriers could provide additional insights into age-associated changes important to disease progression to provide some initial clarity on this issue. …More
I find it interesting that dasatinib was identified in their drug screen. Our upcoming Phase 2 trial “SToMP-AD” will use a combination of dasatinib and quercetin as an approach to clear senescent cells from older adults with amnestic MCI/early AD. In 2015 Dr. Jim Kirkland’s group at the Mayo Clinic, Rochester, Minnesota, identified dasatinib as an agent that selectively clears senescent cells (Zhu et al., 2015). Broadly, we hypothesize that targeting biological aging processes, such as cellular senescence, may delay, prevent, or effectively treat age-associated diseases such as AD. Our trial, along with others, will test this approach.
Identifying dasatinib at the convergence of two independent drug screens, one focused on senescent cells and the other on AD pathogenesis, is an exciting finding that lends support to the overall strategy.
For SToMP-AD, we are using an intermittent dosing schedule that consists of six cycles of 100 mg dasatinib/day x two days followed by a 14-day no-treatment period. This is repeated six times (i.e., subjects receive 1,200 mg dasatinib across the 12-week treatment period). In contrast, an example of a cancer regime would be 140 mg daily for 12 weeks, i.e., patients receive 11,760 mg across the 12-week period. The total difference across 12 weeks is that cancer patients receive 9.8 times more dasatinib than we are using. I am not an oncologist, and while there may be different doses for different stages and types of cancer, the example I provided is FDA-approved for Philadelphia chromosome-positive acute lymphoblastic leukemia, and resistance to, or intolerance of, initial treatment.
References:
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015 Aug;14(4):644-58. Epub 2015 Apr 22 PubMed.
Make a Comment
To make a comment you must login or register.