Can an Old Diuretic Drug Disarm APOE4, Prevent Alzheimer’s?
Quick Links
Repurposing FDA-approved drugs for Alzheimer’s disease has for many years been a dream among researchers. Now this dream has led Yadong Huang and Marina Sirota, University of California, San Francisco, to an unlikely candidate—the diuretic bumetanide. In the October 11 Nature Aging, they reported that this commonly prescribed medicine reverses transcriptomic changes in human and mouse neurons expressing APOE4. Bumetanide cut the number of amyloid plaques in APOE4 knock-in mice, strengthened synapses, and improved memory. Intriguingly, older people taking the drug to reduce edema were less likely to have AD, even after controlling for hypertension. Huang is planning an AD clinical trial, which he hopes to begin in mid-2022.
- Drug repurposing found bumetanide reversed transcription signatures driven by APOE4.
- People over 65 taking this diuretic were less likely to have AD.
- A clinical trial in AD is slated to start next year.
“This is a great example of a precision-medicine approach, using data-driven methodology for proof-of-concept drug repurposing in AD,” Jean Yuan, National Institute on Aging, Bethesda, Maryland, told Alzforum. Zac Gerring, QIMR Berghofer Medical Research Institute, Brisbane, Australia, agreed. “It’s great to see such a comprehensive drug-repurposing study where the authors provide a compelling case for bumetanide in AD,” he wrote to Alzforum (full comment below).
Howard Feldman, University of California, San Diego, also thinks the study is impressive. “The authors have provided this excellent roadmap for repurposing a drug that follows a target-specific approach, whether or not bumetanide eventually fulfills clinical promise,” he wrote to Alzforum (full comment below).
Drug repurposing has been tried before in AD, and never worked. More recently, such research has focused on identifying molecules that alter molecular signatures of cells carrying AD risk genes (Xu et al., 2021; Peng et al., 2020). In this study, the authors focused on APOE4, the strongest genetic risk factor for sporadic AD.
First author Alice Taubes and colleagues began by looking for any AD gene-expression signature that might be driven by ApoE4. They searched a gene-expression database built from cortical transcriptomes of 97 people who had had AD and 116 controls. Compared to E4/4 cognitively healthy controls, carriers with AD had 235 differently expressed genes.
With this E4 AD signature in hand, the scientists went in search of chemicals that would flip it back to a more E3-like state. They turned to another database called the Connectivity Map. Built by researchers at MIT, CMap connects more than 1,300 FDA-approved drugs to transcriptomic changes in cancer cells (Oct 2006 news). “Though not brain cells, they were a starting point,” Sirota said. “Many have argued the Connectivity Map is not appropriate for brain-related diseases, but these results suggest otherwise,” Gerring wrote. Using CMap, he recently identified classes of drugs that change expression modules associated with AD risk alleles (Gerring et al., 2021).
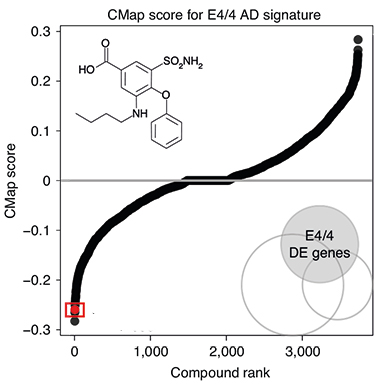
Flipping Drugs. Many of the more than 1,300 drugs (black dots creating S curve) in the Connectivity Map database, up- or downregulated the APOE4 transcriptome in cancer cells. Bumetanide (boxed red dot) reversed the molecular signature. [Courtesy of Taubes et al., Nature Aging, 2021.]
In CMap, the authors looked for drugs that increased expression of downregulated genes in the E4 signature, or decreased expression of upregulated genes. Bumetanide, used to treat edema associated with heart, liver, or kidney failure, was the fourth-most-effective transcriptome-flipping drug (see image at left). Huang said they chose to study bumetanide further because it is safe for long-term use and has evidence of crossing the blood-brain barrier. Feldman noted that the drug has been tested in other brain diseases, such as neonatal seizures and autism, due to its putative GABAergic inhibition (Zhang et al., 2020; Soul et al., 2021; Kharod et al., 2019).
Taubes and colleagues found that bumetanide similarly reversed the signatures of cultured human excitatory, inhibitory, and dopaminergic neurons derived from APOE4/4-induced pluripotent stem cells.
What about in vivo? The researchers treated 16-month-old APOE4/4 knock-in mice with 0.2 mg per kg of the drug for eight weeks. RNA-seq indicated that the APOE4 transcriptome had flipped in many cell types, not just neurons (see image below).

Reversal of Fortune. In bumetanide-treated APOE4/4 mice, brain cells (colored dots) flipped their transcriptional profile. The effect was statistically significant for a majority of cell types (below red dotted line). [Courtesy of Taubes et al., Nature Aging, 2021.]
Aged APOE4 knock-in mice have a memory deficit and weak long-term potentiation (LTP), a proxy for neuron plasticity. Bumetanide reversed these phenotypes. Treated APOE4 knock-ins better remembered where the platform was in a water maze than their untreated counterparts and, in hippocampal slices from treated mice, LTP appeared normal.
To see whether bumetanide affects amyloid load, Taubes crossed APOE4/4 knock-ins with J20 mice, which develop plaques by 6 months of age, then treated 10-month-old offspring with 0.2 mg per kg of bumetanide for 12 weeks. Here, too, the drug flipped transcriptomes of various brain cell types, restored LTP in hippocampal slices, and reduced the number of plaques.
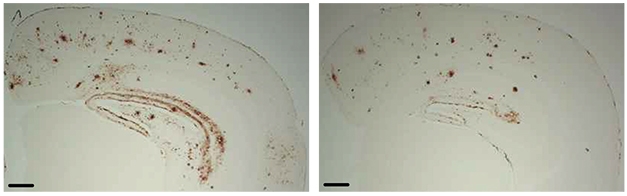
Preventing Plaques. Bumetanide-treated APOE4/J20 mice (right) had fewer amyloid deposits in the hippocampus and cortex than did controls (left). [Courtesy of Taubes et al., Nature Aging, 2021.]
How bumetanide might spur these changes is unclear. The authors do not believe it is simply by reducing plaque load. In both human cells and animal models, the scientists found consistent changes in pathways governing GABAergic signaling, circadian entrainment, and morphine addiction—none of which have any immediate link to plaques. Jeffrey Cummings, University of Nevada, Las Vegas, noted that these pathways are not even commonly implicated in AD. “Bumetanide worked as well in mice with or without amyloid, so its mechanism may not be specific to AD,” he wrote.
To see if these bumetanide effects translate to the real world, the researchers searched electronic health records from the University of California, San Francisco, and the Mount Sinai Health System in New York. They were trying to determine if people taking this drug for other indications are also protected from developing AD. Comparing 3,751 people over 65 who are on the drug with 7,502 age- and sex-matched controls, the researchers found that the prevalence of AD among the former was 35 percent lower in the Californian cohort, and a whopping 75 percent lower in the New York cohort. This finding held when Taubes compared people taking bumetanide with those taking a different diuretic, ruling out mere lowering of blood pressure, a leading risk factor for dementia, as the operative explanation (Aug 2018 conference news).
Huang is talking with clinicians and FDA scientists about testing bumetanide for AD. He hopes to enroll both APOE4/4 and APOE3/4 carriers in a clinical trial to begin next year.—Chelsea Weidman Burke
References
News Citations
- New Database Connects Gene Expression, Disease
- Could Better Blood Pressure Management Preserve Cognition?
Research Models Citations
Paper Citations
- Xu Y, Kong J, Hu P. Computational Drug Repurposing for Alzheimer's Disease Using Risk Genes From GWAS and Single-Cell RNA Sequencing Studies. Front Pharmacol. 2021;12:617537. Epub 2021 Jun 30 PubMed.
- Peng Y, Yuan M, Xin J, Liu X, Wang J. Screening novel drug candidates for Alzheimer's disease by an integrated network and transcriptome analysis. Bioinformatics. 2020 Nov 1;36(17):4626-4632. PubMed.
- Gerring ZF, Gamazon ER, White A, Derks EM. Integrative Network-Based Analysis Reveals Gene Networks and Novel Drug Repositioning Candidates for Alzheimer Disease. Neurol Genet. 2021 Oct;7(5):e622. Epub 2021 Sep 9 PubMed.
- Zhang L, Huang CC, Dai Y, Luo Q, Ji Y, Wang K, Deng S, Yu J, Xu M, Du X, Tang Y, Shen C, Feng J, Sahakian BJ, Lin CP, Li F. Symptom improvement in children with autism spectrum disorder following bumetanide administration is associated with decreased GABA/glutamate ratios. Transl Psychiatry. 2020 Jan 27;10(1):9. PubMed.
- Soul JS, Bergin AM, Stopp C, Hayes B, Singh A, Fortuno CR, O'Reilly D, Krishnamoorthy K, Jensen FE, Rofeberg V, Dong M, Vinks AA, Wypij D, Staley KJ, Boston Bumetanide Trial Group. A Pilot Randomized, Controlled, Double-Blind Trial of Bumetanide to Treat Neonatal Seizures. Ann Neurol. 2021 Feb;89(2):327-340. Epub 2020 Dec 3 PubMed.
- Kharod SC, Kang SK, Kadam SD. Off-Label Use of Bumetanide for Brain Disorders: An Overview. Front Neurosci. 2019;13:310. Epub 2019 Apr 24 PubMed.
External Citations
Further Reading
Primary Papers
- Taubes A, Nova P, Zalocusky KA. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nature Aging, 1, 2021, pp. 932–47. Nat Aging.
- Li Z, Zhao N. A water pill against Alzheimer’s disease. Nature Aging, 1, 2021, pp.868-9. Nat Aging.
Annotate
To make an annotation you must Login or Register.

Comments
QIMR Berghofer Medical Research Institute
Taubes and colleagues identified the sulfamyl diuretic bumetanide, used to treat high blood pressure among other conditions, as a potential drug repurposing candidate for late-onset Alzheimer’s disease. Bumetanide is a plausible candidate, and previous studies have hinted that hypertension treatment could reduce the risk of developing Alzheimer’s disease and other types of dementia.
The drug was identified using a method known as “signature mapping,” where APOE-dependent gene expression signatures of Alzheimer’s disease were integrated with drug-gene signatures from the Broad Institute’s Connectivity Map. Signature mapping basically prioritizes drugs that are predicted to “normalize” a disease signature, assuming this will alleviate disease symptoms and potentially halt onset and progression of disease.
My lab and others have also demonstrated the utility of this approach in Alzheimer’s disease (Gerring et al., 2021).
The functional validation and retrospective clinical record evidence that support bumetanide in Alzheimer’s is particularly impressive. The use of APOE-knock-in mice to not only show bumetanide reverses hallmark pathological changes in Alzheimer’s disease but potentially reverses cognitive deficits is an important first step to argue for human clinical trials.
The replication of the (predicted) Connectivity Map signatures using induced pluripotent stem cell-derived neurons is an important validation of the signature mapping approach. This is particularly important because, as the authors point out, the Connectivity Map signatures are derived from cancer lines, rather than neuronal or glial cell lines. Many have argued the connectivity map is inappropriate for brain-related diseases. These results suggest otherwise. However, I believe the development of a connectivity map for brain-cell types is critical for the discovery of new drug candidates for neurological and psychiatric diseases.
Finally, the use of electronic medical records, while fraught with the limitations of retrospective data analysis (i.e., causality cannot be established due to unobserved confounders), provided additional support for the use of bumetanide in Alzheimer’s disease. The replication of results across two independent medical record databases, combined with efforts to control for hypertension, improves the validity of these results.
Overall, it’s great to see such a comprehensive drug-repurposing study. The authors provide a compelling case for bumetanide in Alzheimer’s disease in addition to an experimental validation of sorts for the use of “signature mapping” in other brain-related diseases.
References:
Gerring ZF, Gamazon ER, White A, Derks EM. Integrative Network-Based Analysis Reveals Gene Networks and Novel Drug Repositioning Candidates for Alzheimer Disease. Neurol Genet. 2021 Oct;7(5):e622. Epub 2021 Sep 9 PubMed.
University of California, San Diego
This study provides a comprehensive and impressive roadmap to develop an evidence base for bumetanide, a loop diuretic, as a repurposed candidate drug for Alzheimer’s disease. Their treatment target was the reversal of characterized ApoE4-dependent transcriptomic signatures. They incorporated the publicly available databases for ApoE genotype-dependent transcriptomic signatures in human brain, and high-throughput screening of more than 1,300 drugs, to see which could reverse these signatures, with bumetanide emerging as the top candidate.
Taking this treatment into ApoE knock-in mice, with and without Aβ accumulation, the authors demonstrate how bumetanide can reverse electrophysiological, cognitive, and behavioral deficits in preclinical animal models and in pluripotent stem cell-derived neurons. They extended their studies with pharmaco-epidemiological investigations of bumetanide from the UCSF and Mount Sinai health systems electron health record databases to identify lower AD prevalence in individuals over age 65 on bumetanide than in those not so treated.
Parenthetically, this medication has previously attracted attention for the treatment of brain diseases, including neonatal seizures and autism spectrum, with some putative GABAergic inhibitory properties. It would be very helpful to know how long it took to complete all the different pieces of this research plan and bring it to its current status.
The authors conclude that these studies support bumetanide as a promising clinical trials candidate for AD. This prospect raises important questions for its future clinical development plan, including the dose selection, or range of doses to be tested, the pharmacodynamic endpoints that will be used in these clinical trials to judge the sufficiency of dose, the strategy for selecting the disease stage to test it in, and the detailed design considerations of the first clinical trials. Whether bumetanide, a potent diuretic with the potential for considerable metabolic side effects in older persons, is going to be viable will likely come down to dose and tolerability.
All these considerations follow the impressive contribution of Taubes et al., who have provided an excellent roadmap for a repurposing drug that pursues a target-specific ApoE4 approach, whether or not it is bumetanide that eventually fulfills the clinical promise.
University of Nevada Las Vegas
This is an interesting and well-done paper. Its strengths include the use of multiple converging lines of evidence, such as big data confirmed in mice and iPSCs, an effect reflected in patient electronic medical records (i.e., reduced AD), and a focus on APOE, an important and neglected therapeutic target.
The study also raises questions. For example, bumetanide worked as well in animals without amyloid as in those with amyloid; so this mechanism may not be specific to AD. The pathways involved—morphine addiction, GABA-ergic pathways, circadian entrainment—are not usually implicated in AD.
Bumetanide is a very strong diuretic that can produce dehydration and electrolyte imbalance. It would be difficult to use in older patients with AD. It may be possible to re-re-engineer the molecule if these pathways are confirmed to be important.
The confirmation of the cell-based and mouse effects in the eHR is encouraging with the following caveats: AD was reduced in patients who had congestive heart failure or other conditions that may not be present in many AD patients, and the exposures in those patients may have been much longer than most trials would allow.
Overall, the observations in this study may apply more broadly than just to AD.
Make a Comment
To make a comment you must login or register.