Are Tauopathies Caused by Neuronal and Glial Senescence?
Quick Links
In Alzheimer’s disease, tau tangles correlate closely with neurodegeneration, but how they work their mischief remains unclear. Two papers now link tau to the buildup of senescent cells in the brain. These aged, sick cells can neither divide nor undergo programmed cell death. Instead, they can stick around for years, and scientists believe they contribute to many chronic diseases of aging. In the September 19 Nature, researchers led by Darren Baker at the Mayo Clinic in Rochester, Minnesota, reported that eliminating senescent cells as they formed prevented later neurodegeneration and cognitive decline in a mouse model of tauopathy. Meanwhile, researchers led by Miranda Orr at the University of Texas Health Science Center, San Antonio, found that destroying senescent cells in mice at advanced stages of tauopathy slowed neurodegeneration and corrected aberrant brain blood flow. That work appeared in the August 20 Aging Cell.
- Eliminating senescent glia in young tauopathy mice prevents neurodegeneration.
- Killing senescent neurons in old tauopathy mice slows degeneration.
- Senescent cells may mediate tau toxicity.
John Trojanowski at the University of Pennsylvania, Philadelphia, said the papers were important for pointing to a new line of investigation. “The findings suggest senescent cells contribute to the initiation and progression of tau-mediated disease,” he wrote to Alzforum (full comment below). Miranda Reed at Auburn University, Alabama, noted that the papers complement each other, with one focusing on prevention and the other on treatment. “Senescence may help to explain why aging is a risk factor for Alzheimer’s disease,” she said.
Relatively few studies have examined senescence in the context of neurodegenerative disease. Some researchers have reported senescent astrocytes and microglia in Alzheimer’s brain, but the effect of these cells was not clear (Flanary et al., 2007; Bhat et al., 2012). However, senescent cells are known to secrete proinflammatory factors and proteases that can damage surrounding tissue (for review see Coppé et al., 2010).
To find out what these cells do in the brain, Baker and colleagues turned to PS19 mice. These animals express high levels of human mutant P301S tau in neurons and have developed tau tangles in the hippocampus by six months of age. In the hippocampi of four-month-old transgenics, before tangles formed, joint first authors Tyler Bussian and Asef Aziz detected twice as many senescent astrocytes and microglia as in age-matched wild-type mice.
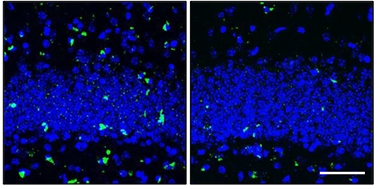
Preventing Pathology.
In the dentate gyrus of the hippocampus (nuclei are blue), PS19 mice (left) accumulate neurofibrillary tangles (green) by eight months of age, but not if senescent cells are pruned as they arise (right). [Courtesy of Nature, Bussian et al.]
The authors used a genetic strategy to selectively eliminate these moribund cells. They crossed PS19 mice with animals that express two complementary pieces of caspase 8 under the control of a promoter that only becomes active in senescent cells. When activated, this protease drives apoptosis, or programmed cell death. To join those caspase fragments together and trigger this process, the authors administered the drug AP20187 twice weekly, beginning at three weeks of age. AP is a small molecule that has a moiety on either end that binds each fragment.
At six months, the authors analyzed the brains of the treated mice and found they resembled wild-type brains. AP treatment had prevented the proliferation of astrocytes and microglia normally seen in PS19 mice. Treated mice had wild-type levels of hyperphosphorylated tau. At eight months, treated mice had much fewer neurofibrillary tangles than untreated controls (see image above). Brain weight and cortical thickness remained at wild-type levels. In addition, the mice recognized a new scent as readily as wild-types did, suggesting preserved memory. All told, the treatment seemed to protect the mice against tau pathology and its effects. The authors used a second strategy as well, giving the cancer drug ABT263 to PS19 mice from three weeks to six months of age. This drug, which kills senescent cells by inhibiting an anti-apoptotic factor, also protected the mice.
The results suggest not only that mutant tau in neurons somehow spurs senescence in astrocytes and microglia, but that sick glia then signal back to neurons, worsening tau pathology in a vicious cycle, Baker said. In an accompanying Nature editorial, Jay Penney and Li-Huei Tsai at the Massachusetts Institute of Technology, Cambridge, said they were surprised that senescent glia could heighten neuronal tau pathology. “[This] indicates complex crosstalk between neurons and senescent glia,” they wrote. Nevertheless, they found the data convincing. “Glial senescence ultimately promotes neuronal degeneration,” they concluded.
This scenario might play out in other neurodegenerative diseases as well. Studies have found that senescent astrocytes in the spinal cord harm motor neurons in mouse models of amotrophic lateral sclerosis (Nov 2014 news).
Wolfgang Streit at the University of Florida, Gainesville, said the findings dovetail with what he has found in the human brain. In postmortem tissue, he sees dystrophic, senescent microglia in regions with a high burden of tau tangles (Streit et al., 2009). These sick microglia can no longer support neurons, and might be a key reason for neurodegeneration, he suggested.

Tackling Tangles.
When treated with senolytic drugs (bottom), rTg4510 mice have fewer tangle-bearing neurons (gold; nuclei are blue) than untreated controls (top). [Courtesy of Aging Cell, Musi et al.]
In her study, Orr focused on neurons rather than glia. Phosphorylated or aggregated tau are known to cause post-mitotic neurons to re-enter the cell cycle. Lacking the wherewithal to complete that, they die via apoptosis (Park et al., 2007; Arendt, 2012; Seward et al., 2013). However, Orr was struck by the observation that neurons containing tangles do not always die. Instead, some persist long-term. She wondered if they hold off death by entering a senescent state.
Joint first authors Joseph Valentine and Nicolas Musi tested this theory by comparing gene-expression data from neurons with tangles and neighboring ones without. To do this, they analyzed an existing data set generated from neurons microdissected from postmortem AD brain (Dunckley et al., 2006). Neurons with tangles had a transcriptome reminiscent of senescent cells, expressing high levels of inflammatory and cell survival genes, and low levels of apoptotic genes. The researchers did not examine glia. “We can’t rule out that other cell types are also senescent,” Orr told Alzforum.
The authors saw a similar pattern in the rTg4510 mouse. These animals overexpress human mutant P301L tau in the forebrain. They develop tau tangles by four months old and begin losing neurons by six. At six months, the authors found high levels of senescent and inflammatory genes in the hippocampus. In addition, they saw evidence of DNA damage and poor mitochondrial function, both of which go along with senescence.
Was mutant tau to blame? The authors compared the rTg4510 mice to animals that overexpress wild-type human tau. Brains from the latter looked like those of wild-type mice, indicating that tau level alone does not cause senescence.
Could amyloid plaques also contribute to senescence? Orr and colleagues tested this in 3xTg AD mice, which develop plaques by six months and, in their hands, tangles by 18. At 12 months of age, no senescent cells appeared, suggesting that plaques by themselves do not drive senescence. In support of the causal role of tau tangles, the authors found senescent cells in postmortem brain tissue from people who had progressive supranuclear palsy, a pure tauopathy. Again, senescence markers correlated with tangle load.
Finally, the authors treated 21-month-old rTg4510 mice with two senolytic drugs, dasatinib and quercetin. The former is FDA-approved for cancer, while the latter is a natural antioxidant. The combination is being tested in clinical trials for other age-related diseases, such as pulmonary fibrosis and kidney disease. The mice were first crossed with mouse tau knockouts to remove endogenous protein. At 24 months, the researchers found 35 percent fewer tangle-bearing neurons in the brains of treated mice than in untreated (see image above). This was accompanied by several improvements in brain health. Treated mice had more gray matter and their ventricles were about 30 percent smaller than controls’. Unlike controls, they had normal cerebral blood flow, and they had half as many white-matter hyperintensities. Levels of synaptic proteins were a third higher than in untreated mice, suggesting neurons were healthier.
Although Orr examined neurons, and Baker glia, Orr said the studies paint a similar picture. “Together, our studies say that many cell types in the brain become senescent during tauopathy, and that clearance of these cells leads to improvements in brain structure and function,” she told Alzforum.
The findings from rTg4510 mice hint that removing senescent cells could improve brain function even at advanced disease stages, Orr said. She hopes senolytic drugs may offer potential for treating AD patients. Other researchers were intrigued by their potential as well, but said more questions need to be answered before human testing. Reed noted that senescent cells that arise after acute trauma seem to play a role in repair, suggesting that eliminating them could have negative consequences. “I wouldn’t try senolytics for prevention or chronic treatment until we understand the long-term consequences of them,” Reed said. Baker also expressed caution, noting that researchers have not yet shown that senescent cells contribute to pathology in human brain.
Writing about these findings in a September 17 JAMA editorial, Tamara Tchkonia and James Kirkland at the Rochester Mayo Clinic, noted that senolytics improve both health and lifespan in aging mice, but agree that more data are needed on potential adverse effects in people. “If senolytics are shown to be safe and effective in humans, they could transform care of older adults and patients with multiple chronic diseases,” they speculated.—Madolyn Bowman Rogers
References
Research Models Citations
News Citations
Paper Citations
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007 Mar;10(1):61-74. PubMed.
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer's disease. PLoS One. 2012;7(9):e45069. PubMed.
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. PubMed.
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009 Oct;118(4):475-85. PubMed.
- Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci. 2007 Mar 14;27(11):2969-78. PubMed.
- Arendt T. Cell Cycle Activation and Aneuploid Neurons in Alzheimer's Disease. Mol Neurobiol. 2012 Apr 13; PubMed.
- Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, Roberson ED, Bloom GS. Amyloid-β Signals Through Tau to Drive Ectopic Neuronal Cell Cycle Re-entry in Alzheimer's Disease. J Cell Sci. 2013 Jan 23; PubMed.
- Dunckley T, Beach TG, Ramsey KE, Grover A, Mastroeni D, Walker DG, LaFleur BJ, Coon KD, Brown KM, Caselli R, Kukull W, Higdon R, McKeel D, Morris JC, Hulette C, Schmechel D, Reiman EM, Rogers J, Stephan DA. Gene expression correlates of neurofibrillary tangles in Alzheimer's disease. Neurobiol Aging. 2006 Oct;27(10):1359-71. PubMed.
External Citations
Further Reading
Primary Papers
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018 Oct;562(7728):578-582. Epub 2018 Sep 19 PubMed.
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018 Dec;17(6):e12840. Epub 2018 Oct 11 PubMed.
- Penney J, Tsai LH. Elimination of senescent cells prevents neurodegeneration in mice. Nature. 2018 Oct;562(7728):503-504. PubMed.
- Tchkonia T, Kirkland JL. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA. 2018 Sep 17; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Pennsylvania
Both these papers are important because they open up new lines for investigating the impact of aging-related neurodegenerative diseases, and we know so little about how mechanisms of aging interact with or contribute to mechanisms of neurodegeneration. Thus, as Bussian et al. note, markers of senescence are present in human neurodegenerative diseases, but the significance of this was not clear. Now, Bussian et al. show that tau Tg mice accumulate p16INK4A-positive senescent astrocytes and microglia and that elimination of these cells genetically and with first-generation senolytic stops gliosis, hyperphosphorylation of both soluble and insoluble tau, neurofibrillary tangles, and neurodegeneration. These findings suggest senescent cells contribute to the initiation and progression of tau-mediated disease. Hence, senescent cells could be a logical target for therapies to treat neurodegenerative disease, but obviously further replication will be needed for these novel results, and this is provided in large part by the second paper by Musi et al. who show that treatment of tau-transgenic mice with senolytics to remove senescent cells also showed a beneficial therapeutic response including reducing tangles, neuron loss, and ventricular enlargement. Together these studies support the notion that senescent cells may play a role in aging-related tauopathies.…More
Make a Comment
To make a comment you must login or register.