VCAM1: Gateway to the Aging Brain?
Quick Links
Remember those eerie research findings evoking “The Twilight Zone”? Mysterious ingredients in the blood of young mice rejuvenated the brains of their elders, while the blood of old mice sped up aging in young’uns. The identity of blood-borne agents of aging remains a mystery, but researchers believe they have at least zeroed in on the agents’ gateway to the brain. At the joint Keystone symposia Advances in Neurodegenerative Disease Research and Therapy / New Frontiers in Neuroinflammation, held June 17–21 in Keystone, Colorado, Tony Wyss-Coray of Stanford University in Palo Alto reported that expression of vascular cell adhesion molecule 1 (VCAM1) on brain endothelial cells was required for old blood to accelerate aging in young brains. In fact, blocking this receptor even slowed classic symptoms of normal brain aging, such as less neurogenesis and more neuroinflammation, in control mice that never received blood transfusions.
- VCAM1 expression on the luminal side of blood endothelial cells rises with age.
- Only with VCAM1 did blood from old mice speed up aging in the brains of young mice.
- Blood from active mice boosted neurogenesis in sedentary ones.
- Alkahest started a Phase 2 trial testing a fraction from young human plasma in people with AD.
The findings cement the role of systemic factors in brain aging and suggest that blocking their lines of communication with the brain could have restorative effects. The therapeutic prospect is especially appealing because treatments targeting VCAM1 would not need to cross the blood-brain barrier, which remains a formidable hurdle for CNS drug development. The researchers uploaded their findings to bioRχiv, where the pdf has been viewed nearly 2,200 times since January (Yousef et al., 2018).
Aging is the strongest risk factor for Alzheimer’s and most other neurodegenerative diseases. Circulating factors play a key role, according to findings over the years by Wyss-Coray and colleagues. Not only did they find that blood from young mice halted synaptic deficits, loss of neurogenesis, and even memory loss in AD mouse models, but blood from old mice quickened these processes in young mice (Aug 2011 news; May 2014 conference news; Sep 2016 conference news).
At Keystone, Wyss-Coray presented new results of efforts to understand how outside factors manage to drive aging inside the brain. Reasoning that the blood-brain barrier must be an active participant, Wyss-Coray and colleagues sifted through plasma proteomics data the lab had generated in years prior. They searched for proteins whose concentration shot up with age, and which were known to be expressed by the brain endothelial cells (BECs) that line the BBB. Of the 31 proteins that went up with age, eight were expressed in BECs, and five of those were implicated in vascular function. Of those five, soluble VCAM1 stood out as the protein that increased most in the plasma with age, Wyss-Coray reported at Keystone.
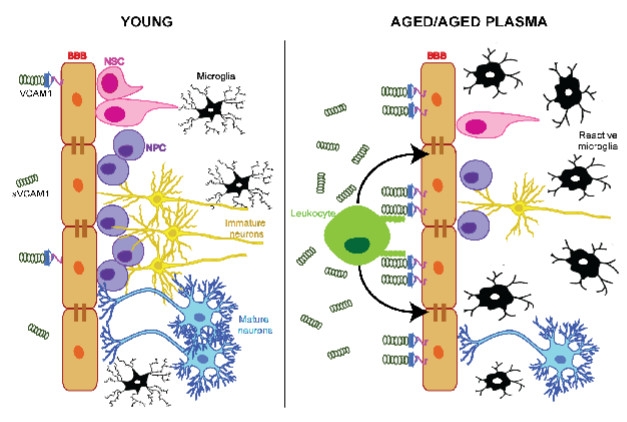
VCAM1 Opens Door to Aging. In the young brain, low levels of VCAM1 on brain endothelial cells allow microglia to remain ramified and calm, and neurogenesis to proceed. In the old brain, these cells increase VCAM1 expression, which tethers more leukocytes to their luminal side. This activates microglia, suppresses neurogenesis. [Courtesy of Yousef et al., bioRχiv, 2018.]
VCAM1 is part of the immunoglobulin receptor family, whose expression rises on many cell types in response to injury. The receptor binds to the integrin VLA4, expressed on leukocytes. The soluble version of VCAM1—sVCAM1—is continually shed by the ADAM17 protease, and its concentration in the blood correlates with cognitive impairment and even mortality, Wyss-Coray said.
To investigate VCAM1 expression on BECs, the scientists infused fluorescently tagged anti-VCAM1 antibodies into mice; this labeled cells expressing the receptor on the luminal side of the cerebrovasculature. They found a patchy, sparse expression pattern. In young mice, at most 4 percent of BECs in hippocampal blood vessels expressed VCAM1, and this rose to about 5 percent as the animals aged. Injecting young mice with lipopolysaccharide boosted VCAM1 expression to 15 percent of BECs, and even more so in older mice. Single-cell RNA sequencing of BECs revealed that VCAM1 expression was limited to BECs in arteries/arterioles and veins/venules; it was not expressed in capillaries. A single-cell transcriptomic study of the brain vasculature, by Christer Betsholtz at Sweden’s Uppsala University, also reported expression of VCAM1 on arteries and veins, but not capillaries (Vanlandewijck et al., 2018).
At Keystone, Wyss-Coray reported that vein/venule BECs expressing VCAM1 also expressed a slew of pro-inflammatory cytokine receptor genes, as well as receptors known to interact with leukocytes.
The researchers next investigated how young versus old plasma affected BEC VCAM1 expression. Exposing young mice to plasma from aged mice—whether via parabiosis or intravenous injection—boosted VCAM1 expression on BECs. Similarly, cultured BECs ramped up VCAM1 expression when treated with plasma from old, but not young, mice. Notably, the researchers found the same result when treating young mice or cultured BECs from young mice with aged human plasma. This suggested that shared factors in aged mouse and human plasma ramped up VCAM1 expression on BECs.

Stop VCAM1, Stop Aging? Anti-VCAM1 antibodies increase the number of proliferating (EdU+) neural progenitor cells (Sox2+) in the subventricular zone (white lines) of the dentate gyri of young mice treated with aged human plasma (left two panels). Anti-VCAM1 antibodies decrease the number of activated (CD68+) microglia (Iba1+) (right two panels). [Courtesy of Yousef et al., bioRχiv, 2018.]
So far so good, but does VCAM1 facilitate what aged plasma does to the brain? To find out, the researchers injected an anti-VCAM1 antibody into young animals treated with plasma from old mice or humans. As seen previously, in young brains, aged plasma reduced proliferation of neural progenitor cells, a marker of neurogenesis, and boosted activation of microglia; however, treatment with the VCAM1 antibody completely blocked these effects. The same was true in the context of normal aging, as 16-month-old mice injected with the antibody every three days for three weeks had higher rates of neurogenesis and fewer reactive microglia than untreated mice.
Does the VCAM1 need to be on BECs? To find out, the researchers generated mice in which VCAM1 expression can be shut off only in BECs. Doing so between the age of two and 16 months resulted in old mice having more neurogenesis and less neuroinflammation than did mice that had been expressing VCAM1 on BECs all along.
The findings suggest that VCAM1 expression on BECs somehow mediates the detrimental effects of aged plasma on the brain, Wyss-Coray told the audience. It is unclear how this works, but Wyss-Coray hypothesized that systemic factors ramp up VCAM1 expression on the brain endothelium, and possibly also VLA-4 expression on circulating leukocytes. This would lead to leukocyte tethering along the luminal side of the BBB, where they might inflame the endothelium and transmit inflammatory mediators into the brain. Either directly or via activation of microglia, these mediators could suppress neurogenesis. The exact sequence of events remains to be discovered.
Fielding questions from the audience after his talk, Wyss-Coray added nuance to his findings. He said that using an anti-VLA4 antibody instead of an anti-VCAM1 antibody prevented the age-related increase in microglial activation but not the decline in neurogenesis. When asked whether a VCAM1 antibody might work as an anti-aging therapeutic, Wyss-Coray cautioned that anti-VLA4 antibodies, which are a standard treatment for people with multiple sclerosis and Crohn’s disease, cause cancer in a small proportion of patients. Given the severity of those diseases, the cancer risk is considered acceptable. “VCAM1 antibodies would probably be seen in a similar light: If they are efficacious against AD or a similar neurodegenerative disease, a black label might be accepted, and the potential cancer risk would be carefully monitored,” he told Alzforum later.
Jonas Neher of the German Center for Neurodegenerative Diseases in Tübingen was intrigued by the work. Neher recently reported that systemic inflammation steers the course of future microglial responses to neurodegenerative disease in the brain (Apr 2018 news). However, Neher contends that soluble factors, such as cytokines, are highly likely to play a role in signaling into the brain. Wyss-Coray agreed, adding that soluble factors could still influence the brain via leukocytes, by upregulating both VCAM1 on the endothelium and VLA-4 on leukocytes.
‘Fit’ Infusions, Youthful Fractions
While the new data on VCAM1 speak to how old blood ages the brain, the Keystone meeting also featured findings about how young blood, perhaps even that of athletes, rejuvenates. Zurine de Miguel, a senior scientist in Wyss-Coray’s lab, reported that not just youth but physical fitness might bestow neurogenesis powers onto blood. De Miguel found that plasma collected from mice with access to a running wheel and infused into sedentary mice boosted the birth of newborn neurons in them. Previous studies have linked exercise to neurogenesis, and De Miguel’s findings now suggest that factors in the plasma facilitate the benefit. De Miguel also reported that so-called “runner’s plasma” boosted expression of neuroplasticity genes in recipients, and dampened their inflammatory response to lipopolysaccharide. De Miguel demurred when asked to opine whether regular infusions with the blood of exercisers could substitute for actually working out.
Eva Czirr of Alkahest, a biotech company in San Carlos, California, presented clinical findings based on Wyss-Coray’s research. He co-founded Alkahest with Karoly Nikolich in hopes of parlaying plasma products into treatments for neurodegenerative and other age-related diseases. The company completed a small Phase 1 study testing the safety of “young plasma” derived from 18- to 22-year-old donors in recipients with mild to moderate AD (Dec 2017 conference news).
As that trial was ongoing, Czirr and colleagues zeroed in on a plasma fraction—dubbed GRF6019—that they speculate will contain the beneficial properties of whole plasma without its downsides. Comprising about 400 proteins, GRF6019 is devoid of immunoglobulins and clotting factors; this eliminates the need to match recipients to donors and reduces the risk of stroke. Plasma fractions are made from pooled plasma of many donors, facilitating production of large, standardized batches. Alkahest acquires its plasma fractions from Barcelona-based Grifols, the world’s largest producer of blood products. Czirr said that Grifols collects an average of 25,000 plasma donations per day around the world.
At Keystone, Czirr reported that GRF6019 had lasting benefits in old mice. Three months after receiving intravenous infusions, the mice still had elevated rates of neurogenesis, lower markers of neuroinflammation, and performed better on tests of memory than their untreated counterparts.
In addition, Czirr reported that Alkahest’s Phase 2 clinical trial is officially underway (clinicaltrials.gov). The trial tests GRF6019 in 40 participants with clinically diagnosed mild to moderate AD. They will be equally randomized to receive a low or high dose of GRF6019 intravenously for five consecutive days on the first week of the trial, then again for five days during week 13. The primary outcome measure is safety; cognitive, functional, and neuropsychological tests make up secondary outcomes. Participants will be monitored for a total of six months during the trial.—Jessica Shugart
References
News Citations
- Paper Alert: Do Blood-Borne Factors Control Brain Aging?
- In Revival of Parabiosis, Young Blood Rejuvenates Aging Microglia, Cognition
- Young Blood a Boon for APP Mice
- Stuck in the Past? Microglial Memories Dictate Response to Aβ
- Blood, the Secret Sauce? Focus on Plasma Promises AD Treatment
Therapeutics Citations
Paper Citations
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018 Feb 14; PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
University of Edinburgh
The concept of “VCAM-1 opening the door to aging” is very interesting and fascinating. Aging has been associated with a low‐grade inflammatory status of the immune system, commonly called “inflammaging,” a contraction of inflammation and aging (Franceschi et al., 2000; Franceschi and Bonafè, 2003), in which pro‐inflammatory cytokines (e.g., IL‐6, TNF and IL‐1β) are key players in unhealthy aging. Inflammaging is one of the most important etiological factors in age‐related neurodegenerative diseases, as “neuro‐inflammaging” is associated with neurodegeneration and decreased cortical volume (Yankner et al., 2008). With aging, both macrophages and microglia display impaired and prolonged activation to insults, reduced motility, and impaired phagocytosis (Rawji et al., 2016). This overactivation induces reactive oxygen species production and attracts peripheral leucocytes, thereby altering the metabolic and trophic support that glial cells normally provide to their environment (Chinta et al., 2015). Impaired phagocytosis results in increased toxic protein accumulation, which is associated with progressive pathology of Aβ and α‐synuclein in Alzheimer’s and Parkinson’s diseases, respectively (Spittau et al., 2017). Although still not fully understood, the impact of chronic inflammation on brain functions is suspected to be harmful and to favor the progression of cognitive impairment associated with normal aging (Liang et al., 2017).…More
Importantly, inflammation associated with aging can be detected using in vivo molecular magnetic resonance imaging (MRI) of VCAM-1 (Montagne et al., 2012). The high sensitivity of intravenous paramagnetic microparticles of iron oxide (MPIOs) targeting VCAM-1 allowed us to non-invasively reveal cerebrovascular inflammation in aged (24-month-old) compared to young (3-month-old) Swiss “healthy” mice. In aged mice, there were significantly more signal voids induced by MPIOs-αVCAM-1 than in young mice (11.4 percent vs 4.2 percent of the whole brain, respectively; Montagne et al., 2012, Figure 8D,E). Using the same approach, elevated levels of endothelial activation were confirmed in aged mice (18-month-old) in both the brain and kidney (Belliere et al., 2015). Longitudinal monitoring of this chronic endothelial activation could be useful to evaluate the effectiveness of therapeutic interventions (such as anti-inflammatory treatment, anti-hypertensive drugs, statins, specific diets, etc.) to prevent age-associated cognitive impairment.
References:
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244-54. PubMed.
Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003 Apr;31(2):457-61. PubMed.
Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41-66. PubMed.
Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016 Mar;139(Pt 3):653-61. Epub 2016 Jan 29 PubMed.
Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2015 Aug;68:3-7. Epub 2014 Oct 1 PubMed.
Spittau B. Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front Aging Neurosci. 2017;9:194. Epub 2017 Jun 14 PubMed.
Liang Z, Zhao Y, Ruan L, Zhu L, Jin K, Zhuge Q, Su DM, Zhao Y. Impact of aging immune system on neurodegeneration and potential immunotherapies. Prog Neurobiol. 2017 Oct;157:2-28. Epub 2017 Aug 4 PubMed.
Montagne A, Gauberti M, Macrez R, Jullienne A, Briens A, Raynaud JS, Louin G, Buisson A, Haelewyn B, Docagne F, Defer G, Vivien D, Maubert E. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage. 2012 Nov 1;63(2):760-70. Epub 2012 Jul 17 PubMed.
Belliere J, Martinez de Lizarrondo S, Choudhury RP, Quenault A, Le Béhot A, Delage C, Chauveau D, Schanstra JP, Bascands JL, Vivien D, Gauberti M. Unmasking Silent Endothelial Activation in the Cardiovascular System Using Molecular Magnetic Resonance Imaging. Theranostics. 2015;5(11):1187-202. Epub 2015 Aug 8 PubMed.
Make a Comment
To make a comment you must login or register.