Surprise! TMEM106b Fibrils Found in Neurodegenerative Diseases
Quick Links
When David Eisenberg’s group set out to solve the structure of TDP-43 fibrils from the human brain, the UCLA scientists had no idea they were about to stumble upon a different fibril entirely. Eisenberg’s team extracted plentiful fibrils from the brains of people who had died with frontotemporal lobar degeneration with TDP-43 pathology, yet, to their surprise, they weren't made of TDP-43. Instead, the isolated filaments turned out to be of none other than TMEM106b, a lysosomal protein with known genetic ties to FTD.
- Three cryo-EM studies identified similar TMEM106b fibrils.
- The fibrils were detected across neurodegenerative diseases and in aged controls.
- Fibrils consisted of a cleaved C-terminal fragment of this lysosomal protein.
The Eisenberg group wasn’t alone. Two others—one led by Michel Goedert and Sjors Scheres at the MRC Laboratory of Molecular Biology in Cambridge, England, the other by Anthony Fitzpatrick at Columbia University in New York—had also plucked TMEM106b fibrils from the brains of people with FTLD-TDP, and from people who had died with other neurodegenerative diseases. The Cambridge researchers even detected the fibrils in the brains of older people who had died without any sign of neurodegeneration.
In three papers published in March, the scientists introduce similar cryo-electron microscopy structures of TMEM106b filaments. The protofilament cores consisted of a 135-residue, C-terminal fragment of the transmembrane protein—a domain that typically dangles into the lysosomal lumen. Importantly, to twist into this shape, the domain must be shed from the lysosomal membrane. The structural details reported by each group varied somewhat, but all three converged on a strikingly similar protofilament: a meandering contortion consisting of 17-19 β-strands, which wind into fibrils made of either one or two protofibrils.

TMEM106b Protofilament Flavors. Cross-sections of TMEM106b filaments, showing either one (left) or two (right) protofilaments featuring the same fold. [Courtesy of Schweighauser et al., Nature, 2022.]
That the C-terminal twists into fibrils may explain why these aggregates have gone undetected for so long—most previous studies have used N-terminal antibodies to label the protein.
The findings raise more questions than answers, scientists pointed out. Crucially, researchers want to know how, or even if, TMEM106b fibrils contribute to neurodegenerative disease. Are they toxic in their own right, or do they goad aggregation of other proteins? What triggers their formation? How does TMEM106b fibrillization relate to lysosomal dysfunction? All these questions remain on the table at this point, but floodgates are sure to open.
“These striking findings will completely reset research on TMEM106b and its involvement in FTLD as a risk/protective factor,” said Christian Haass of the German Institute for Neurodegenerative Diseases in Munich. Both protective and risk variants of the gene are known. Answering the myriad new questions raised by these studies will also illuminate how lysosomal function is disturbed in FTLD and probably other neurodegenerative diseases, he added.

TMEM106b Across Proteinopathies. Fibrils of TMEM106b form following cleavage of the protein's lumenal portion. The fibrils were seen in multiple proteinopathies; one study even found them in healthy older people. [Courtesy of Chang et al., Cell, 2022.]
Because Eisenberg and colleagues had been fishing for TDP-43 fibrils, their study, published March 28 in Nature, was initially limited to brain samples from patients with FTLD-TDP. Once first author Yi Jiang and colleagues discovered that they had isolated TMEM106b rather than TDP-43, they started a detailed structural analyses of four brain samples, each harboring a purportedly different morphological flavor of TDP-43 pathology, i.e., subtypes A, B, C, and D. Five subtypes, A to E, of FTD-TDP-43 have been described.
The authors identified three ultrastructural polymorphs of TMEM106b fibrils, all sharing the same core protofilament fold. All three polymorphs were found in all four donors. Polymorph 1, which represented about 80 percent of the fibrils across the samples, comprised a single protofilament. A pair of protofilaments with different symmetries made up the other two polymorphs. The common core consisted of residues 120-254 of TMEM106b, with the fragment’s N-terminus nestled deep within the center of the fold.
Over the course of 18 strands, the fragment wrapped around itself, with the C-terminus of the protofilament core meeting up with the N-terminus. The researchers likened the fold to an 18-hole golf course. “These 18 β-strands vary in length (from 3 to 15 residues) and curvature as do fairways,” they wrote. “Moreover, the N- and C-terminal strands are near each other, just as the first and 18th holes in a golf course adjoin near the clubhouse.”

Fore! In what the authors describe as a “golf-course fold,” the C-terminus of TMEM106b arranges into 18 β-strands, with the N-terminus of the fragment nestled deep within the center of the fold (left). These protofilaments stack upon each other (right). [Courtesy of Jiang et al., Nature, 2022.]
The final 19 residues of TMEM106b were excluded from the fibril core, suggesting they may have formed a so-called “fuzzy coat” projecting out of the ordered fibril. These coats have been found in fibrils of tau and Aβ as well (Apr 2017 conference news).
As amyloid fibril structures go, 18 β-strands rank above par for a protofilament core. Aβ, tau, TDP-43, and α-synuclein protofibrils comprise five, eight, 10, and 12 strands, respectively. Tightly fused together with multiple steric zippers, the 18 β-strands triple the stability of the average fibril, the researchers reported. This remarkable stability suggests that once formed, TMEM106b fibrils are unlikely to unfurl.
Jiang and colleagues detected no fibrils made of TDP-43 in any of the brain samples. They did find amorphous aggregates of the RNA binding protein in their sample preps. This led them to conclude that TDP-43 aggregates exist in a predominantly non-fibrillar form, while TMEM106b is the major fibrillar species in brain samples from people with FTLD-TDP.
This latter conclusion seems at odds with a recent study led by Benjamin Ryskeldi-Falcon of the MRC in Cambridge, who also co-authored the Cambridge group’s current TMEM106b study. Last year, Falcon reported a double-spiral fold of TDP-43 fibrils extracted from the motor and frontal cortices of people who had died with FTD-ALS (Dec 2021 news).
Where were these TDP-43 fibrils in the samples used in Eisenberg’s study? Eisenberg believes the discrepancy could come down to the different neuropathological conditions of the donors—namely, FTLD in his study versus FTLD-ALS in Falcon’s study. Falcon disagreed, noting that in both neurological conditions, TDP-43 inclusions amass within the frontal cortex, a region where Falcon detected TDP-43 fibrils and Eisenberg did not. Falcon favors the idea that the explanation lies in fibril isolation procedures, which varied markedly between the two groups. For one, the Cambridge group used ultracentrifugation to sediment the fibrils, while the UCLA group used tabletop centrifuges. Falcon believes that a faster spin, coupled with milder detergents, is the key to pulling down TDP-43 fibrils, which are less stable and dense as TMEM106b fibrils.
Regardless of whether TDP-43 fibrils were there or not, Falcon, Goedert, Scheres, and colleagues said they started noticing a mysterious type of fibril lurking in their brain sample preps from multiple types of proteinopathies more than a year ago. Scheres told Alzforum that the extensive glycosylation pattern of the mystery fibril gave away its identity, since TMEM106b is known to be heavily glycosylated. Ultimately, the amino acid sequence deciphered from the cryo-EM structure of the fibrils confirmed that they were composed of residues 120-254 of TMEM106b.
In their study, reported back-to-back with Eisenberg’s in Nature, first author Manuel Schweighauser and colleagues used cryo-EM to visualize the structure of these fibrils isolated from various brain regions of 22 people with different types of neurodegenerative diseases. The list included sporadic and familial Alzheimer’s disease (AD), people with substantial Aβ plaques who were cognitively normal at death, corticobasal degeneration, sporadic and familial FTLD-TDP and FTLD-tau, argyrophilic grain disease, limbic-predominant neuronal inclusion body four-repeat tauopathy, aging-related tau astrogliopathy, sporadic and familial Parkinson’s disease, dementia with Lewy bodies, multiple system atrophy, and amyotrophic lateral sclerosis. These authors also found TMEM106b fibrils in three people who had died without a neurological disorder and little to no accumulation of Aβ, tau, α-synuclein, or TDP-43 in their brains.
From all these samples, the researchers detected a total of three protofilament folds. Fold 1 was more common than folds 2 or 3. Each sample contained only one type of fold. All three folds shared a similar ordered core consisting of 17 β-strands wrapped into five β-sheets.
These folds stacked differently with each other. Fold 1 formed two fibrils made of either one or two protofilaments. Folds 2 and 3 only layered into single protofilament fibrils. The structure of the first 46 residues was shared by all folds, while the middle and C-terminal portions differed slightly. Importantly, the researchers found that fold types did not track with neurodegenerative disease type. Furthermore, genotyping revealed an equal representation of both the protective (S185) and risk (T185) alleles of TMEM106b across the 22 sample donors, suggesting that fibrils could form from either variant.
Because the researchers detected TMEM106b fibrils in three controls, they wondered whether they might form as people age. To address this without going through the trouble of cryo-EM, they raised an antibody, TMEM239, against residues 239-254, and used it to probe brain samples from 16 neurologically normal people who ranged from 20 to 101 years old at death. While most TMEM106b antibodies bind to the N-terminus of the protein, the researchers wanted to label the C-terminal fragment to measure its cleavage and, potentially, its aggregation. By immunoblot, TMEM239 detected a 29kDa band in the sarkosyl-insoluble fraction. The authors believe it represents the glycosylated C-terminal fragment of TMEM106b, which weighs in at 17kDa, unmodified. This insoluble fragment was not detected in anyone under the age of 46, but consistently cropped up in people in their 70s or older. The finding suggests that aggregation of the C-terminal fragment is an age-related phenomenon.
In brain sections, TMEM239 labeled inclusions that Scheres described as “a big blob in the cytoplasm.” These occurred in people who had had neurodegenerative disease, and also in older, but not younger, controls. Because the epitope that TMEM239 binds is suspected to be buried deep within the TMEM106b fibril core, it is unclear what species of the protein the antibody is labeling within these inclusions, Scheres and Goedert said. However, they pointed out that the epitope could be exposed during the denaturation of the sample during western blot. For immunohistochemistry, fixation of the tissue could expose the epitope, or perhaps the antibody is binding to partially formed fibrils, Goedert suggested. Also, the fact that the antibody labels inclusions rather than healthy lysosomes, and nothing in young controls, suggests it is binding an aggregated species of the protein and is not merely an artefact, they told Alzforum.
Fitzpatrick and colleagues published their paper on March 4 in Cell. Co-first authors Andrew Chang, Xinyu Xiang, Jing Wang, Carolyn Lee, Tamta Arakhamia, Marija Simjanoska, and colleagues used both cryo-EM and mass spectrometry to discern the structure of TMEM106b fibrils isolated from the brains of 11 people with different neurodegenerative diseases, including eight with FTLD-TDP, two with progressive supranuclear palsy, and one with DLB. They found the fibrils—once again composed of residues 120-254 of TMEM106b—in all these brain samples. However, they did not detect the fibrils in controls, nor did they find them in every case of PSP or DLB that they examined. In contrast, all cases of FTD-TDP that they examined had the fibrils, including one person who died in their 50s.
Chang and colleagues detected one common protofilament core, arranged into fibrils made of either one or two protofilaments. The ratio of singlet to doublet fibrils varied from 1:2 to 2:1 across the samples. The researchers identified 19 β-strands in the common protofilament fold. Scheres and Eisenberg found 17 and 18, respectively. However, the scientists agreed that the calling of individual β-strands is somewhat subjective, and that the main TMEM106b protofilament folds reported by each group are essentially the same.
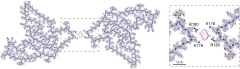
Anionic Fibril Starter? In doublet fibrils of TMEM106b, protofilaments interact via an anionic intermediate. The identity of this non-proteinaceous matchmaker is unknown, but researchers speculate it could facilitate fibrillization. [Courtesy of Chang et al., Cell, 2022.]
Similar to reports by the other groups, Chang found that, in the doublet fibril, the two protofilaments were arranged with a twofold symmetry, and interacted via a mysterious middleman—a non-protein, anionic cofactor that mingled with the sidechains of K178 and R180 in both protofilaments. This cofactor also associated with K178 in singlet fibrils. The researchers speculated that it, along with other post-translational modifications such as glycosylation, could promote fibril formation. The acidic environs of the lysosome may also have something to do with fibrillization of TMEM106b following its cleavage by a protease. Its identity is unknown.
Using a polyclonal antibody specific for the C-terminus of TMEM106b to probe their brain samples via immunoblot, the scientists in Fitzpatrick’s lab detected bands corresponding to TMEM106b monomers, as well as higher-order oligomers. They found that these oligomeric forms were more abundant in brain samples from people who had died with a neurodegenerative disease than in samples from controls. To the authors, this argues against the idea that TMEM106b fibrillization is merely an age-related phenomenon, suggesting instead that the fibrils play a part in neurodegenerative disease.
Further work is required to explain why only the Cambridge group managed to detect TMEM106b fibrils via cryo-EM in non-pathological controls, and to determine whether the fibrils are harmful, irrelevant, or even protective against disease, wrote Hideyuki Takahashi and Stephen Strittmatter of Yale University in New Haven, Connecticut, in an accompanying editorial in Nature. “Given that the risk of developing a neurodegenerative disease increases with age, and that TMEM106B fibrils might accumulate with age, it is possible that the presence of TMEM106B fibrils enhances mechanisms of neurodegeneration driven by other factors and protein aggregates,” they wrote. “Going forward, research priorities should include determining the prevalence of TMEM106B fibrils and their effect on endolysosomal cell biology and on neurodegenerative diseases that involve other types of fibril.”

Havoc in the Lysosome? Fitzpatrick et al. hypothesize that cleavage of TMEM106b and its subsequent fibrillization could result from, and then exacerbate, dysfunction of the lysosome. Fibrils could form inside or outside of the lysosome following leakage, and may even goad aggregation of other proteopathic scourges. [Courtesy of Chang et al., Cell, 2022.]
Scientists from all groups agreed that the proteolytic cleavage of TMEM106b is a critical event leading to its fibrillization. What dictates that cleavage, including the identity of the protease, remains unknown. It is possible that lysosomal stressors—including age or even decreasing levels of progranulin—might rev TMEM106b cleavage and fibrillization, Fitzpatrick et al. suggested. TMEM106b fibril accumulation could then have myriad downstream effects, worsening lysosomal function and goading the aggregation of other proteins including TDP-43, tau, and α-synuclein in suspectable individuals, they wrote. Furthermore, fibrillization of TMEM106b would compromise the function of TMEM106b itself, further burdening the lysosome. Leaky lysosomes could explain how aggregates of TMEM106b wound up in the cytosol.
“Currently we only know that TMEM106b forms intracellular inclusions. It remains unclear if they occur in late endosomes/lysosomes, or are distributed throughout the entire cell body, maybe even due to rupture of these organelles,” commented Haass. “Importantly, we do not even know yet if these filaments are associated with disease, or are directly causative. They may still be rather innocent bystanders, like lipofuscin.”
Peter Nelson of the University of Kentucky in Lexington doesn’t think so. He led the characterization of limbic predominant TDP-43 encephalopathy (LATE), a neuropathological disorder that rises with age and is now tied to cognitive decline. He suspects that, similar to inclusions of TDP-43, fibrils of TMEM106b are unlikely to be benign. “Whenever you have a genetic association with neurodegenerative disease, then you’re probably onto something upstream in the cascade to dementia,” he said. The clinical-pathological correlations of TMEM106b fibrils need to be ironed out, he said. Perhaps TMEM106b aggregation plays a part in the vast clinical and pathological variation manifested by FTD, LATE, and other neurodegenerative proteinopathies, he added.
To Ralph Nixon of New York University in Orangeburg, the findings focus attention squarely on lysosomal dysfunction as a common, primary culprit driving different neurodegenerative diseases. Akin to his work implicating the β-CTF fragment of amyloid precursor protein (APP) as a lysosomal menace, Nixon raised the possibility that fragments of TMEM106b could exert a neurotoxic effect, and perhaps their aggregation is a neuroprotective response. Nixon noted that recent studies suggest that TMEM106b binds to lysosomal vATPases, triggering a rise in lysosomal pH that leads to a host of other problems for the cell (Jul 2017 news; Sep 2020 news).
David Holtzman of Washington University in St. Louis also suspects TMEM106B fibrils are toxic in some way. “Combined with prior data showing that a polymorphism in TMEM106b alters risk in FTLD-TDP-43 due to progranulin mutations, the new findings make it likely that the aggregation of TMEM106b into an amyloid conformation is directly linked to pathogenesis of not only FTLD-TDP-43 but also other diseases where it forms amyloid,” he wrote to Alzforum. “These important findings should now lead to new studies to understand what prompts TMEM106b to aggregate, what the consequences are in animal and cellular models, and how to prevent its aggregation.”—Jessica Shugart
References
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Chang A, Xiang X, Wang J, Lee C, Arakhamia T, Simjanoska M, Wang C, Carlomagno Y, Zhang G, Dhingra S, Thierry M, Perneel J, Heeman B, Forgrave LM, DeTure M, DeMarco ML, Cook CN, Rademakers R, Dickson DW, Petrucelli L, Stowell MH, Mackenzie IR, Fitzpatrick AW. Homotypic fibrillization of TMEM106B across diverse neurodegenerative diseases. Cell. 2022 Apr 14;185(8):1346-1355.e15. Epub 2022 Mar 4 PubMed.
- Schweighauser M, Arseni D, Bacioglu M, Huang M, Lövestam S, Shi Y, Yang Y, Zhang W, Kotecha A, Garringer HJ, Vidal R, Hallinan GI, Newell KL, Tarutani A, Murayama S, Miyazaki M, Saito Y, Yoshida M, Hasegawa K, Lashley T, Revesz T, Kovacs GG, van Swieten J, Takao M, Hasegawa M, Ghetti B, Spillantini MG, Ryskeldi-Falcon B, Murzin AG, Goedert M, Scheres SH. Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature. 2022 May;605(7909):310-314. Epub 2022 Mar 28 PubMed.
- Jiang YX, Cao Q, Sawaya MR, Abskharon R, Ge P, DeTure M, Dickson DW, Fu JY, Ogorzalek Loo RR, Loo JA, Eisenberg DS. Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Nature. 2022 May;605(7909):304-309. Epub 2022 Mar 28 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Biomedizinisches Centrum (BMC), Biochemie & Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE)
What a surprise! Nowadays, one would assume that rather simple pathological abnormalities, such as intracellular amyloid filaments, should have been seen by neuropathologists for a long time. Not so. Suddenly, three independent publications pop up and report the widespread existence of intracellular filaments composed of TMEM106b in many neurodegenerative diseases, as well as normal and pathological aging.
It is unlikely that these filaments are artifactually generated by the extraction protocols, as they are formed in a strictly age-dependent manner. But why were they not seen before? One obvious possibility is that detection of TMEM106b fibers requires antibodies to the luminal region, which selectively accumulates within the deposits, and which were not used in previous research.…More
These striking findings will completely reset research on TMEM106b and its involvement in FTLD as a risk/protective factor. TMEM106b is well-known in the field, since it has been genetically associated with FTLD-TDP caused by PGRN haploinsufficiency (Van Deerlin et al., 2010).
We found some time ago that TMEM106b is a type 2 intramembrane protein, which is predominantly located within late endosomes and lysosomes (Lang et al., 2012). The protein undergoes regulated intramembrane proteolysis similar to the amyloid precursor protein (Brady et al., 2014). Shedding of the ectodomain occurs by an unknown protease, most likely a lysosomal protease, whereas the membrane-retained N-terminal stub is removed by intramembrane cleavage executed by Signal Peptide Peptidase-like 2 (SPPL2a) (Brady et al., 2014), an intramembrane cleaving protease of the GxGD type (Steiner et al., 2000).
As for Aβ-peptide generation, proteolytic processing appears to be a prerequisite for TMEM106b fiber formation, since all three manuscripts consistently report a fibrillar core encompassing roughly amino acids 120 to 254. TEMEM106b fibers are found intracellularly.
Strikingly, we and many others reported that PGRN deficiency is associated with lysosomal dysfunction (Götzl et al., 2016; Götzl et al., 2018). One could therefore speculate that endosomal/lysosomal TMEM106b is deposited in these organelles during aging and disturbs their physiological function. Alternatively, dysfunctional lysosomes could induce deposition and thus accelerate secondary malfunction. Increased expression and shedding should then drive the disease, a hypothesis that can now easily be checked. A first hint may come from the finding that TMEM106b increased in an age-dependent manner in PGRN knockout animals and exacerbated lysosomal dysfunction (Zhou et al., 2017; Götzl et al., 2014).
However, currently we only know that TMEM106b forms intracellular inclusions. It remains unclear if they occur in late endosomes/lysosomes, or are distributed throughout the entire cell body, maybe even due to rupture of these organelles. Importantly, we do not even know yet if these filaments are associated with disease, or are directly causative. They may still be rather innocent bystanders, like lipofuscin.
So as always, an important discovery raises a lot of new questions. Answering them may allow us to finally understand the functional TMEM106b/PGRN interconnection, and the mechanism of how lysosomal function is disturbed in FTLD and probably other neurodegenerative diseases as well.
References:
Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL, Ferrer I, Lladó A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, Lopez de Munain A, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, Dekosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, Decarli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tuñón MT, Martínez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010 Mar;42(3):234-9. PubMed.
Lang CM, Fellerer K, Schwenk BM, Kuhn PH, Kremmer E, Edbauer D, Capell A, Haass C. Membrane Orientation and Subcellular Localization of Transmembrane Protein 106B (TMEM106B), a Major Risk Factor for Frontotemporal Lobar Degeneration. J Biol Chem. 2012 Jun 1;287(23):19355-65. PubMed.
Brady OA, Zhou X, Hu F. Regulated intramembrane proteolysis of the frontotemporal lobar degeneration risk factor, TMEM106B, by signal peptide peptidase-like 2a (SPPL2a). J Biol Chem. 2014 Jul 11;289(28):19670-80. Epub 2014 May 28 PubMed.
Steiner H, Kostka M, Romig H, Basset G, Pesold B, Hardy J, Capell A, Meyn L, Grim ML, Baumeister R, Fechteler K, Haass C. Glycine 384 is required for presenilin-1 function and is conserved in bacterial polytopic aspartyl proteases. Nat Cell Biol. 2000 Nov;2(11):848-51. PubMed.
Götzl JK, Lang CM, Haass C, Capell A. Impaired protein degradation in FTLD and related disorders. Ageing Res Rev. 2016 May 7; PubMed.
Götzl JK, Colombo AV, Fellerer K, Reifschneider A, Werner G, Tahirovic S, Haass C, Capell A. Early lysosomal maturation deficits in microglia triggers enhanced lysosomal activity in other brain cells of progranulin knockout mice. Mol Neurodegener. 2018 Sep 4;13(1):48. PubMed.
Zhou X, Sun L, Brady OA, Murphy KA, Hu F. Elevated TMEM106B levels exaggerate lipofuscin accumulation and lysosomal dysfunction in aged mice with progranulin deficiency. Acta Neuropathol Commun. 2017 Jan 26;5(1):9. PubMed.
Götzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, Janssens J, van der Zee J, Lang CM, Kremmer E, Martin JJ, Engelborghs S, Kretzschmar HA, Arzberger T, Van Broeckhoven C, Haass C, Capell A. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014 Mar 12; PubMed.
Washington University
This series of three papers clearly shows that amyloid fibrils composed of the C-terminal region of TMEM106b are abundant in FTLD-TDP-43 as well as during aging and some other neurodegenerative disorders. Combined with prior data showing that a polymorphism in TMEM106b alters risk in FTLD-TDP-43 due to progranulin mutations, the new findings make it likely that the aggregation of TMEM106b into an amyloid conformation is directly linked to pathogenesis of not only FTLD-TDP-43 but also other diseases where it forms amyloid.
This important set of findings should now prompt new studies to understand what leads TMEM106b to aggregate, what the consequences are in animal and cellular models, and how to prevent TMEM106b aggregation.…More
Penn Neurological Institute
These are really exciting findings from structural biology, and they open up many questions about TMEM106B. It's fantastic that multiple groups are seeing similar things, since that helps the field, especially those of us who do not know that much about cryo-EM.
The first thing I considered is why we had not seen TMEM106B in inclusions from neuropathological specimens, since our group had stained many brain sections in 2013 (Busch et al., 2013). I think the answer here is that we used an N-terminus antibody. At that time, there were no commercial antibodies against TMEM106B that worked well for much, so we had raised antibodies to both C-terminus and N-terminus epitopes, but the N-terminus antibody much more clearly worked. It looks like these TMEM106B species are C-terminus. …More
The second thing I considered is whether the data from these papers agrees with "clues" we had from other lines of investigation. The exciting thing is that they do. First, many groups, including ours, have seen evidence for a dimer form of TMEM106B, as well as for glycosylation of TMEM106B, both of which are in line with the Fitzpatrick group paper's findings.
It will be important to understand what the role of missense variants in TMEM106B, glycosylation of TMEM106B, dimerization of TMEM106B, is in driving the formation of fibrils. It seem very possible that increased expression of TMEM106B, which we have linked to specific FTD-associated genetic polymorphisms through a CTCF-mediated mechanism (Gallagher et al., 2017), may make fibrillization events more likely.
Moreover, the fact that TMEM106B fibrils might be found in synucleinopathies and in FTD, but not in AD, accords with our clinical finding that TMEM106B genotypes associate with rate of decline in synucleinopathy and in FTD, but not in AD (Tropea et al., 2019).
All in all: an exciting time to be a TMEM106B researcher!
References:
Busch JI, Martinez-Lage M, Ashbridge E, Grossman M, Van Deerlin VM, Hu F, Lee VM, Trojanowski JQ, Chen-Plotkin AS. Expression of TMEM106B, the frontotemporal lobar degeneration-associated protein, in normal and diseased human brain. Acta Neuropathol Commun. 2013 Jul 11;1(1):36. PubMed.
Gallagher MD, Posavi M, Huang P, Unger TL, Berlyand Y, Gruenewald AL, Chesi A, Manduchi E, Wells AD, Grant SF, Blobel GA, Brown CD, Chen-Plotkin AS. A Dementia-Associated Risk Variant near TMEM106B Alters Chromatin Architecture and Gene Expression. Am J Hum Genet. 2017 Oct 16; PubMed.
Tropea TF, Mak J, Guo MH, Xie SX, Suh E, Rick J, Siderowf A, Weintraub D, Grossman M, Irwin D, Wolk DA, Trojanowski JQ, Van Deerlin V, Chen-Plotkin AS. TMEM106B Effect on cognition in Parkinson disease and frontotemporal dementia. Ann Neurol. 2019 Jun;85(6):801-811. PubMed.
Florey Instotute of Neurosciece and Mental Health
University of Melbourne
It’s surprising to see three very similar papers on TMEM106B appear at the same time.
First impressions on these findings from the sarkosyl pellets from a diverse series of postmortem brain specimens raise questions about whether this is a new type of intraneuronal amyloid. The classical definition of an amyloid, i.e., Congo red negative birefringence, does not appear to have been met. Rather, we are presented with a neuronal cytoplasmic fibrillar aggregate that is (not always) associated with autofluorescent lipofuscin (Schweighauser et al.). These fibrillar aggregates are not specific for a particular disease, but are clearly age-related.
All the fibrils were extracted using sarkosyl extraction protocols, albeit with some variation in the stage of sarkosyl addition: (I) in homogenate before the first centrifugation step (Jiang et al. and Schweighauser et al.) or (II) later, after low-speed centrifugation (Chang et al.). It was argued that the protocol change from II to I was essential for detecting abundant TMEM106B filaments.…More
None of these studies observed fibrils of TDP-43, which have been identified before in ALS/FTD (Arseni et al., 2021) or even in FTD-TDP (Nonaka et al., 2013) using the preferred sarkosyl extraction protocol (I) (sarkosyl in homogenate). However, abundant non-filamentous aggregates of TDP-43 were identified by Jiang et al. in FTD-TDP extracts.
Clearly, there’s still a lot of work to do to determine the biologic and pathologic significance of these TMEM106B fibrillar aggregates. If they are simply the end-stage detritus of age-related lysosomal processing, akin to lipofuscin, then it’s going to be difficult for them to get traction in the field. TDP-43 (whether or not in an aggregated fibrillar state) still remains of great interest, because of its FTD/ALS disease-association status.
References:
Arseni D, Hasegawa M, Murzin AG, Kametani F, Arai M, Yoshida M, Ryskeldi-Falcon B. Structure of pathological TDP-43 filaments from ALS with FTLD. Nature. 2022 Jan;601(7891):139-143. Epub 2021 Dec 8 PubMed.
Nonaka T, Hasegawa M. Prion-like properties of assembled TDP-43. Curr Opin Neurobiol. 2020 Apr;61:23-28. Epub 2019 Dec 18 PubMed.
Cornell University
These represent breakthrough findings in the field.
During the past decade, TMEM106B has been associated with brain aging, myelination disorder, and many neurodegenerative diseases. The demonstration by three independent studies of a TMEM106B C-terminal fragment as the amyloid fibril component raises many interesting questions to be answered.
1) How much TMEM106B protein is cleaved during the disease state, and what is the effect on TMEM106B function?
2) What triggers the cleavage of TMEM106B in the lysosomal lumen and aggregation of the C-terminal fragment?
3) Are TMEM106B fibrils extracellular or inside of the lysosome?
4) What are the effects of TMEM106B fibril formation on lysosomal function and aggregation of other proteins?…More
Ultimately, these answers will help us understand the association of TMEM106B with brain aging and neurodegeneration.
University of Tübingen and DZNE AG Neumann
These are fantastic papers illustrating the immense potential, but also potential caveats to be considered in the interpretation, of cryo-EM in neurodegenerative disease research.
All three papers identified similar fibrils of C-terminal fragments of TMEM106b in the brains of TDP-43 proteinopathy cases. While Jiang et al. did not detect TMEM106 fibrils in their control samples, suggesting specificity for TDP-43 proteinopathies, Schweighauser et al. and Chang et al. identified TMEM106b fibrils also in a wide variety of other neurodegenerative conditions, as well in older neurologically healthy controls, thereby providing sufficient evidence that TMEM106b aggregation seems to be rather a common event in neurodegenerative conditions and upon aging.…More
These findings raise crucial questions that will keep the field busy for a while, e.g.:
(i) What are the neuropathological correlates of the TMEM106b aggregates in brain sections? Which cells develop aggregates? Association with lipofuscin? Association with inclusions composed of TDP-43, tau, synuclein in the distinct conditions?
(ii) Mechanism of TMEM106b cleavage and aggregation?
(iii) Functional consequences of TMEM106b fibrils (loss of function/gain of toxic function/neither?).
(iv) Link and role of TMEM106b fibril formation in the pathogenesis of the various neurodegenerative diseases (relevant/irrelevant?).
Specifically in the context of TDP-43 proteinopathies, I think these studies clearly emphasize the future need to modulate/compare extraction methods and to carefully pay attention to the used sample preparation for cryo-EM analysis.
While these three papers could not identify TDP-43 fibrils in their preparations, a previous study by Arseniy et al. did report the presence of TDP-43 filaments by using a slightly different extraction method, raising the possibility that TDP-43 filaments might have been lost in the preparation process in the three TMEM106b papers. In that context, it might be worth recalling that TDP-43 aggregates in the human TDP-43 proteinopathies are not stained with amyloid dyes such as Thioflavin-S (in contrast to inclusions composed of tau, synuclein, Aβ). Therefore it seems plausible that extraction protocols for detection of TDP-43 filaments might have to be adjusted.
I am very much looking further not only to the answers to the questions raised above on TMEM106β, but also to additional cyro-EM studies dissecting TDP-43 aggregates.
References:
Arseni D, Hasegawa M, Murzin AG, Kametani F, Arai M, Yoshida M, Ryskeldi-Falcon B. Structure of pathological TDP-43 filaments from ALS with FTLD. Nature. 2022 Jan;601(7891):139-143. Epub 2021 Dec 8 PubMed.
Make a Comment
To make a comment you must login or register.