Poly Dipeptide in Cerebrospinal Fluid Marks C9ORF72 Expansion Carriers
Quick Links
Run-on repeats of a hexanucleotide sequence in the C9ORF72 gene are the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). According to a March 29 study in Science Translational Medicine, a poly dipeptide produced by these expanded repeats infiltrates the cerebrospinal fluid of expansion carriers, even before symptoms strike. Led by Leonard Petrucelli at the Mayo Clinic in Jacksonville, Florida, the paper’s 79 authors reported that levels of CSF poly(GP) varied widely between carriers, but held steady in each person throughout the course of their disease. Dieter Edbauer of the German Center for Neurodegenerative Diseases in Munich told Alzforum that soon-to-be published data from his lab agree. “Poly-GP in the CSF may be an excellent trait marker [for expansion carriers], rather than a state marker for C9ORF72 disease,” he said.
Petrucelli and colleagues also reported that antisense oligonucleotides aimed at the C9ORF72 expansions triggered a reduction in poly(GP) in patient cells, and in the brains and CSF of mouse models. “This important study demonstrates that poly(GP) proteins in cerebrospinal fluid (CSF) can serve as a biomarker to gauge the effectiveness of specific treatments for C9ORF72-related ALS/FTD, such as antisense oligonucleotides (ASOs) that target GGGGCC repeat RNA,” commented Fen-Biao Gao of University of Massachusetts Medical School in Worcester.
Within the first intron of the C9ORF72 gene, the GGGGCC sequence repeats up to 30 times in healthy people, but hundreds to thousands of times in people with ALS and/or FTD. Sense and antisense transcripts from the expansions aggregate into foci that sequester crucial RNA binding proteins. Dipeptide repeats translated from these toxic transcripts also aggregate and wreak havoc in neurons (for review, see Gendron and Petrucelli, 2017). Animal studies have indicated that both toxic entities can be taken down using antisense oligonucleotides (ASO) aimed at the expansions (Apr 2016 news), a strategy that has already found success in a recent trial for spinal muscular atrophy (Nov 2016 news). Phase 1 trials for C9-targeted ASO therapy have yet to begin for ALS, but are expected in the near future.
During such trials, it would be ideal to know if ASOs engage C9ORF72 expansions and reduce the production of dipeptide repeats in patients, researchers agree. To that end, Petrucelli and colleagues previously developed an antibody-based assay to detect poly(GP)—the most soluble of the five different dipeptide repeats translated from the expansions. Based on findings from a small number of samples, the researchers reported that expansion carriers had significantly more CSF poly(GP) than controls (Aug 2014 news).
To further validate this biomarker, co-first authors Tania Gendron, Jeannie Chew, and Jeannete Stankowski in Jacksonville and Lindsey Hayes of Johns Hopkins University in Baltimore tested larger numbers of expansion carriers and controls over time, including both asymptomatic and symptomatic carriers. Colleagues from multiple institutions extracted CSF from 227 volunteers. Samples were then analyzed at Mayo, and came from 83 C9-ALS patients (including 12 with both ALS and FTD), 24 expansion carriers diagnosed with diseases other than ALS (including FTD and AD), and 120 people with a normal number of repeats—deemed “non-carriers.” Of those non-carriers, 57 had ALS, four had FTD, 10 had AD, one had primary lateral sclerosis, and 48 were healthy controls.
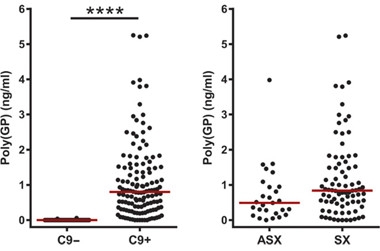
Pumping out Poly(GP).
C9ORF72 expansion carriers have more Poly(GP) in their CSF than non-carriers (left). Symptomatic carriers (SX) tend to have more (right) than asymptomatic (ASX). [Courtesy of Gendron et al., Science Translational Medicine, 2017.]
Levels of poly(GP) were significantly higher in expansion carriers, though concentrations varied by as much as fivefold. While poly(GP) trended higher in symptomatic carriers compared to carriers without symptoms, the difference was not significant. The researchers also tracked poly(GP) longitudinally in 33 expansion carriers, including nine asymptomatic and 24 with ALS or ALS-FTD. Surprisingly to Gendron, levels appeared stable over six to 18 months. Further, among carriers the researchers found no significant correlations between poly(GP) and disease onset, duration, symptom severity, disease type, or any behavioral impairment tested. However, women had lower levels than men.
Why poly(GP) levels vary so much among expansion carriers is unclear. While varying expression levels of repeat DNA may explain it, the researchers are also investigating the length of the repeats. Longer polypeptides host more antibody binding sites, and thus could boost the poly(GP) signal, Petrucelli said. However, the researchers also pointed out that in a clinical trial, each patient would serve as his or her own control, with poly(GP) measured before and after treatment, so comparisons between patients would not be crucial.
As to why levels held steady in each patient over time, the researchers pointed out that poly(GP) is likely produced by many neurons in the brain, of which only a small subset degenerate. Therefore, while poly(GP) released by dead motor neurons may increasingly contribute to the total as the disease progresses, it would be unlikely to rise above the steady production in neurons throughout the brain, the researchers reasoned. This would also explain its detection in asymptomatic carriers, they said.
For CSF poly(GP) to serve as a useful pharmacodynamic marker in ASO clinical trials, it would need to reflect target engagement. To test this, the researchers turned to patient cells. They cultured both lymphoblastoid cell lines generated from patient blood samples as well as neurons differentiated from induced pluripotent stem cells (iPSCs) derived from patient fibroblasts. The dipeptide repeats appeared in lysates from both and also in the culture media, indicating that the cells somehow secreted it. Extracellular poly(GP) correlated with the amount found inside the cells. Finally, the researchers treated lymphoblasts and neuronal cells with ASOs targeting the first intron of C9ORF72. These nucleotides were chemically modified to promote digestion of C9ORF72 RNA via endogenous RNAse H. In response, poly(GP) in the culture media dropped, as did the number of RNA foci and aggregates of dipeptide repeats in the cells.
To test the ASOs in vivo, Gendron and colleagues turned to mice developed by Petrucelli’s lab, which express 66 hexanucleotide repeats from an adeno-associated virus that is injected into the brain. By six months of age, the animals’ neurons are chock-full of RNA foci and dipeptide repeat inclusions. The researchers injected 500μg of C9-ASOs into the right ventricle of the brains of four- to 4.5-month-old animals. Eight weeks later, the mice had fewer repeat-containing mRNAs, RNA foci, and dipeptide inclusions throughout the brain than did PBS-treated controls. CSF poly(GP) in the CSF dropped in parallel, correlating with reduced expansion transcripts and reduced levels of other dipeptide repeats they encode. These findings suggested that measurement of CSF poly(GP) in patients might reflect suppression of the hexanucleotide expression by potential therapeutics.
Aaron Gitler of Stanford University called the study comprehensive and thorough, adding that the longitudinal data support the idea that poly(GP) will serve as a durable pharmacodynamic marker during clinical trials. “As ASOs move into the clinic, this marker will allow researchers to truly test the hypothesis that lowering C9 dipeptides will modify disease,” he told Alzforum. David Corey of University of Texas Southwestern Medical Center in Dallas added that a detectable decrease in poly(GP) during Phase 1 clinical trials could accelerate the initiation of larger Phase 2 and 3 studies
Biogen plans to initiate a Phase 1 trial of ASOs in C9-ALS in the near future, but whether it will measure CSF poly(GP) is still uncertain, according to Petrucelli and co-author Jeffrey Rothstein of Johns Hopkins. Rothstein said that plans might firm up after April.—Jessica Shugart
References
News Citations
- Paper Alert: Could Antisense Therapy One Day Squelch Toxic Repeats in ALS or FTD?
- Positive Trials of Spinal Muscular Atrophy Bode Well for Antisense Approach
- Drug and Biomarker Candidates for C9ORF72 ALS and FTD
Research Models Citations
Paper Citations
- Gendron TF, Petrucelli L. Disease Mechanisms of C9ORF72 Repeat Expansions. Cold Spring Harb Perspect Med. 2017 Jan 27; PubMed.
Further Reading
Papers
- Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, Ostrow LW, Rothstein JD, Troncoso JC, Ranum LP. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):E4968-77. Epub 2013 Nov 18 PubMed.
Primary Papers
- Gendron TF, Chew J, Stankowski JN, Hayes LR, Zhang YJ, Prudencio M, Carlomagno Y, Daughrity LM, Jansen-West K, Perkerson EA, O'Raw A, Cook C, Pregent L, Belzil V, van Blitterswijk M, Tabassian LJ, Lee CW, Yue M, Tong J, Song Y, Castanedes-Casey M, Rousseau L, Phillips V, Dickson DW, Rademakers R, Fryer JD, Rush BK, Pedraza O, Caputo AM, Desaro P, Palmucci C, Robertson A, Heckman MG, Diehl NN, Wiggs E, Tierney M, Braun L, Farren J, Lacomis D, Ladha S, Fournier CN, McCluskey LF, Elman LB, Toledo JB, McBride JD, Tiloca C, Morelli C, Poletti B, Solca F, Prelle A, Wuu J, Jockel-Balsarotti J, Rigo F, Ambrose C, Datta A, Yang W, Raitcheva D, Antognetti G, McCampbell A, Van Swieten JC, Miller BL, Boxer AL, Brown RH, Bowser R, Miller TM, Trojanowski JQ, Grossman M, Berry JD, Hu WT, Ratti A, Traynor BJ, Disney MD, Benatar M, Silani V, Glass JD, Floeter MK, Rothstein JD, Boylan KB, Petrucelli L. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci Transl Med. 2017 Mar 29;9(383) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
DZNE
Critics will argue that detection of poly-GP in the CSF of presymptomatic C9orf72 carriers by Gendron et al. and us (Lehmer et al., 2017) shows that DPR proteins play no role in C9ORF72 pathogenesis. In contrast, both groups of authors argue that expression of poly-GP (and presumably other DPR proteins) contributes to the morphological changes and measurable behavioral abnormalities detected in C9ORF72 carriers many years before meeting the diagnostic criteria for ALS or FTD (Rohrer et al., 2015). Regardless of the exact role in pathogenesis, measurement of poly-GP in the CSF is an excellent marker for repeat-directed therapeutic approaches. Gendron et al. use several in vitro and in vivo assays to convince us that poly-GP in the CSF represents DPR load in patients, because it seems to result from secretion of living cells rather than from passive release from dead neurons. Both reports use a similar MSD immunoassay. In the long run, our assay using only monoclonal antibodies could be more reproducible as a standardized test.
Both papers find that poly-GP in CSF does not correlate with disease severity, age of onset, cognition, or other meaningful disease parameters. Gendron et al. find a non-significant increase in symptomatic versus asymptomatic patients and significantly higher poly-GP levels in male patients. In our smaller cohort, symptomatic and asymptomatic patients show very similar poly-GP levels, but female patients show ~1.7x higher signal than males (p=0.08). It’s puzzling that both cohorts show quite large, unexplained variability in poly-GP levels in C9ORF72 carriers, with several patients having very low poly-GP levels. It is possible that more sensitive single-molecule detection methods will show a clearer picture. Our analysis of poly-GP in control groups revealed an unexpected poly-GP signal in some AD but not in PD patients. So far, we cannot tell whether this signal in patients without C9ORF72 repeat expansion in peripheral blood derives from somatic mosaicism, other poly-GP coding repeat expansions, or (most likely) cross-reactivity with other CSF proteins. Next, we will analyze whether these poly-GP positive AD patients represent a meaningful disease subgroup.
Taken together, poly-GP in the CSF is an excellent trait marker rather than a state marker for C9ORF72 disease that will be useful to address target engagement in future therapeutic trials as shown in a mouse model by Gendron et al. Additionally, neurofilaments should be measured to evaluate whether antisense therapy also restores axonal integrity (Lehmer et al. in press; Weydt et al., 2016; Mattson et al., 2017).
References:
Lehmer C, Oeckl P, Weishaupt JH, Volk AE, Diehl-Schmid J, Schroeter ML, Lauer M, Kornhuber J, Levin J, Fassbender K, Landwehrmeyer B, German Consortium for Frontotemporal Lobar Degeneration, Schludi MH, Arzberger T, Kremmer E, Flatley A, Feederle R, Steinacker P, Weydt P, Ludolph AC, Edbauer D, Otto M. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med. 2017 Jul;9(7):859-868. PubMed.
Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, van Minkelen R, Rombouts SA, Cardoso MJ, Clegg S, Espak M, Mead S, Thomas DL, De Vita E, Masellis M, Black SE, Freedman M, Keren R, MacIntosh BJ, Rogaeva E, Tang-Wai D, Tartaglia MC, Laforce R Jr, Tagliavini F, Tiraboschi P, Redaelli V, Prioni S, Grisoli M, Borroni B, Padovani A, Galimberti D, Scarpini E, Arighi A, Fumagalli G, Rowe JB, Coyle-Gilchrist I, Graff C, Fallström M, Jelic V, Ståhlbom AK, Andersson C, Thonberg H, Lilius L, Frisoni GB, Pievani M, Bocchetta M, Benussi L, Ghidoni R, Finger E, Sorbi S, Nacmias B, Lombardi G, Polito C, Warren JD, Ourselin S, Fox NC, Rossor MN. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015 Mar;14(3):253-62. Epub 2015 Feb 4 PubMed.
Weydt P, Oeckl P, Huss A, Müller K, Volk AE, Kuhle J, Knehr A, Andersen PM, Prudlo J, Steinacker P, Weishaupt JH, Ludolph AC, Otto M. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016 Jan;79(1):152-8. Epub 2015 Dec 17 PubMed.
Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017 May 1;74(5):557-566. PubMed.
UT Southwestern
It is important to understand whether a drug is having the intended effect on its target as soon as possible after initiating experiments. A favorable result showing a decrease in poly(GP) proteins during a Phase 1 clinical trial would encourage starting the much more expansive Phase 2 and Phase 3 trials that are needed to evaluate whether a drug is effective in patients.
In addition, Petrucelli and colleagues show that the poly(GP) marker can be reduced using antisense oligonucleotides (ASOs) that block expression of mutant C9ORF72. An ASO designed to treat spinal muscular atrophy was recently approved, demonstrating the potential of this class of drug to function in the central nervous system. Petrucelli’s results indicate that a similar strategy may be feasible for ALS. The findings smooth the way for clinical trials by suggesting poly(GP) proteins may be a convenient marker of such activity.
Make a Comment
To make a comment you must login or register.