Paper Alert: Could Antisense Therapy One Day Squelch Toxic Repeats in ALS or FTD?
Quick Links
If toxic RNAs are a problem, then antisense oligonucleotides (ASOs) appear to be a solution. In this case, the toxic RNAs are transcribed from hexanucleotide repeats in the C9ORF72 gene, which can cause both amyotrophic lateral sclerosis and frontotemporal dementia in people (see Sep 2011 news). Transgenic mouse models expressing these repeats develop the same RNA foci and the repeat dipeptides the foci generate, as do people with the C9ORF72 mutation, report researchers in the May 4 Neuron. What’s more, treating the mice with antisense oligonucleotides alleviated the pathology, find the scientists led by senior authors Clotilde Lagier-Tourenne of Massachusetts General Hospital in Charlestown and Don Cleveland at the University of California in San Diego (UCSD). Alzforum previewed this work in coverage from the RNA Metabolism in Neurodegenerative Disease symposium held October 15-16, 2015 (see Oct 2015 news; Nov 2015 news).
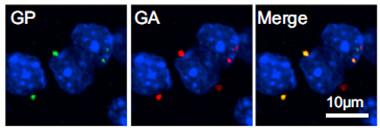
Undesirables.
Aggregates containing poly-glycine-proline (green) and poly-glycine-alanine (red) encoded by C9ORF72 repeats dot cells in the cortex of model mice. [Courtesy of Neuron, Jiang et al.]
Joint first authors Jie Jiang and Qiang Zhu of UCSD, and Tania Gendron of the Mayo Clinic in Jacksonville, Florida, generated two kinds of mice—knockout and overexpression. As others have reported, they, too, observed a general inflammatory condition featuring huge spleens, but no neurodegeneration, in the knockout (see Mar 2016 news).
The researchers made four overexpression lines using exons 1-5 of the C9ORF72 obtained from a patient who had the repeat expansion. Since the repeats tend to expand and contract during the cloning process in bacteria, the authors ended up with one set of mice that make C9ORF72 RNA with about 110 repeats and three others producing varying amounts of the transgene with approximately 450 repeats. The repeats are more stable once in mice. The 450-repeat lines produced repeat RNA foci and repeat dipeptides in their brains within a few months of birth, with more foci and peptides in the higher expressers (see image above). The peptide inclusions multiplied and grew bigger as the animals aged. The higher-expressing 450-repeat mice began behaving oddly once they hit a year old. They struggled to navigate mazes and appeared anxious, rushing to bury unfamiliar marbles. They lost hippocampal neurons as they aged, too.
To see if they could reverse this phenotype, the authors injected antisense oligonucleotides into different parts of the gene or near the repeats themselves. They provided just one intraventricular injection to three-month-old 450-repeat mice, then sacrificed them four weeks later. Using repeat-specific oligos, the researchers were able to eliminate the repeat-containing C9ORF72 RNA while leaving the wild-type version alone.
The treated animals had fewer RNA and peptide aggregates than mice treated with a control, nonspecific ASO. When the authors treated nine-month old mice, the ASOs again reduced RNA and peptide accumulations compared to control animals, within two weeks. The ASOs also alleviated the spatial problems and anxiety in animals sacrificed six months later. At this point, repeat RNA levels were similar to controls, but levels of dipeptide repeats were lower than in animals treated with a control ASO. “These efforts identify gain of toxicity as a central disease mechanism caused by repeat-expanded C9ORF72 and establish the feasibility of ASO-mediated therapy,” the authors wrote.—Amber Dance
References
News Citations
Further Reading
Papers
- Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, Qiu J, Sun Y, Ling SC, Zhu Q, Polymenidou M, Drenner K, Artates JW, McAlonis-Downes M, Markmiller S, Hutt KR, Pizzo DP, Cady J, Harms MB, Baloh RH, Vandenberg SR, Yeo GW, Fu XD, Bennett CF, Cleveland DW, Ravits J. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4530-9. Epub 2013 Oct 29 PubMed.
- Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang J, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron. 2013 Oct 16;80(2):415-28. PubMed.
- Fernandes SA, Douglas AG, Varela MA, Wood MJ, Aoki Y. Oligonucleotide-Based Therapy for FTD/ALS Caused by the C9orf72 Repeat Expansion: A Perspective. J Nucleic Acids. 2013;2013:208245. Epub 2013 Nov 17 PubMed.
- Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, Yachnis AT, Ranum LP. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 2016 May 4;90(3):521-34. Epub 2016 Apr 21 PubMed.
- Riboldi G, Zanetta C, Ranieri M, Nizzardo M, Simone C, Magri F, Bresolin N, Comi GP, Corti S. Antisense Oligonucleotide Therapy for the Treatment of C9ORF72 ALS/FTD Diseases. Mol Neurobiol. 2014 May 9; PubMed.
- Wood H. Neurodegenerative disease: C9orf72 RNA foci--a therapeutic target for ALS and FTD?. Nat Rev Neurol. 2013 Dec;9(12):659. Epub 2013 Nov 26 PubMed.
- Mizielinska S, Isaacs AM. C9orf72 amyotrophic lateral sclerosis and frontotemporal dementia: gain or loss of function?. Curr Opin Neurol. 2014 Oct;27(5):515-23. PubMed.
Primary Papers
- Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis-Downes M, Seelman A, Stauffer JE, Jafar-Nejad P, Drenner K, Schulte D, Chun S, Sun S, Ling SC, Myers B, Engelhardt J, Katz M, Baughn M, Platoshyn O, Marsala M, Watt A, Heyser CJ, Ard MC, De Muynck L, Daughrity LM, Swing DA, Tessarollo L, Jung CJ, Delpoux A, Utzschneider DT, Hedrick SM, de Jong PJ, Edbauer D, Van Damme P, Petrucelli L, Shaw CE, Bennett CF, Da Cruz S, Ravits J, Rigo F, Cleveland DW, Lagier-Tourenne C. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron. 2016 May 4;90(3):535-50. Epub 2016 Apr 21 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.