No Skin Off Your Nose: New Way to Diagnosing Parkinson’s?
Quick Links
As with many neurodegenerative diseases, Parkinson’s is often misdiagnosed. Biomarkers can make the diagnosis more accurate, but existing options such as brain scans and spinal taps are expensive or invasive. Now, two new studies suggest that a small circle of skin, punched out with a handheld tool, could provide a quick and reliable way of detecting the α-synuclein deposits that mark this disease.
• In Parkinson’s patients, nerves in the skin contain α-synuclein seeds
• Two different assays, RT-QuIC and PMCA, can detect their presence
• In small studies, the assays diagnosed the disease with up to 99 percent accuracy
In the September 28 JAMA Neurology, researchers led by Wen-Quan Zou at Case Western Reserve University in Cleveland, Ohio, reported that the nerves of the skin contain α-synuclein seeds that can be amplified by techniques such as real-time quaking-induced conversion (RT-QuIC) or protein-misfolding cyclic amplification (PMCA). Both tests reliably distinguished 67 people with synucleinopathies from 64 age-matched controls, and from 30 people with tauopathies. In the September 22 Movement Disorders, researchers led by Anumantha Kanthasamy at Iowa State University, Ames, described similar findings, with RT-QuIC accurately separating 25 PD cases from 25 controls.
“Seeding assays, such as RT-QuIC and PMCA, when applied to skin biopsies, appear to have great potential as accurate, inexpensive and relatively non-invasive diagnostic methods for Parkinson’s disease, and perhaps other synucleinopathies as well,” Thomas Beach at Banner Sun Health Research Institute in Sun City, Arizona, wrote to Alzforum. Beach co-authored Kanthasamy’s paper.
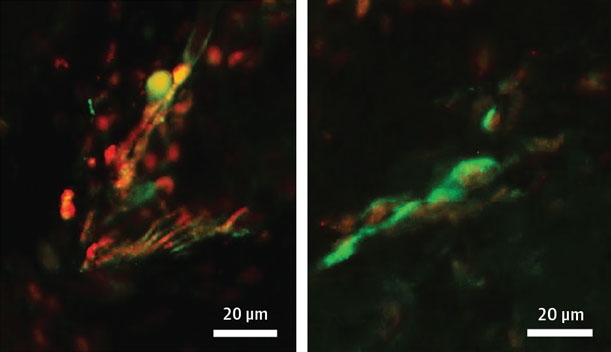
Synuclein in the Skin. Nerve endings (green) in the skin of PD patients (left) contain phosphorylated α-synuclein (red; overlay looks yellow), while those in healthy controls (right) do not. [Courtesy of Wang et al., JAMA Neurology.]
RT-QuIC was developed by Byron Caughey at the National Institutes of Health in Hamilton, Montana, and PMCA by Claudio Soto at University of Texas Medical School at Houston (Orrú et al., 2011; Jun 2001 news). For both methods, researchers add aggregation-ready protein monomers to a donor sample suspected of containing aggregated seeds. If present, the seeds spur the rapid formation of aggregates large enough to visualize with dyes or antibodies (Dec 2016 news). Originally used to detect misfolded prions, the methods have been adapted to amplify Aβ and α-synuclein seeds from cerebrospinal fluid or saliva (Mar 2014 news; Apr 2019 conference news).
Could skin punches offer an easier assay than CSF? Nerve endings in skin contain phosphorylated α-synuclein, hinting at the presence of aggregated protein (May 2015 conference news; Donadio et al., 2017; Rodriguez-Leyva et al., 2017). To test this idea, first author Zerui Wang at Case Western Reserve used RT-QuIC to analyze 4 x 4 mm frozen skin samples taken from the abdomens of cadavers during autopsy. The technique distinguished 47 PD cases from 43 age-matched controls with 94 percent sensitivity and 98 percent specificity. The PMCA assay performed similarly but was slightly less sensitive, at 83 percent. Notably, skin taken from the head outperformed belly skin in a small sample set: for scalp samples, RT-QuIC separated 20 PD cases from 10 controls with perfect accuracy (see image below).

Clean Separation. The RT-QuIC assay amplifies seeds from the scalps of PD samples, but not from controls. [Courtesy of Wang et al., JAMA Neurology.]
What’s in This Skin: Tau or α-Synuclein?
These amplification methods distinguished between synucleinopathies and tauopathies. This is important, because both proteopathies can cause movement disorders that look similar to the clinician. In an autopsy cohort, RT-QuIC separated three synucleinopathies (47 PD, seven dementia with Lewy bodies, three multiple system atrophy) from three tauopathies (17 Alzheimer’s disease, eight progressive supranuclear palsy, five corticobasal degeneration) with 93 sensitivity and 92 percent specificity. The low specificity was due to five AD cases that read as positive. Lewy bodies are present in many cases of AD. Again, PMCA was slightly less sensitive than RT-QuIC. Overall, RT-QuIC had an accuracy of 0.97, and PMCA, 0.94.
Would the techniques work as well on living patients? Wang and colleagues tested skin samples from the neck that were donated by 20 PD patients and 21 age-matched controls, and found that RT-QuIC separated the groups with 95 percent sensitivity and 100 percent specificity, for an accuracy of 0.995. As before, PMCA was a little lower. Zou noted that most people prefer a skin punch to a spinal tap. Patients were given a local anesthetic at the site before the sample was taken.
For their part, Kanthasamy and colleagues found much the same thing. First author Sireesha Manne analyzed autopsy skin samples from the scalp of 25 PD cases and 25 controls by RT-QuIC, which separated them with 96 percent sensitivity and specificity.
As in Wang’s study, these samples had been frozen, but not fixed. To examine whether tissue preparation makes a difference, Manne tested 12 PD and 12 control samples that had been fixed in formalin and embedded in paraffin. Indeed, fixation blunted accuracy, yielding only 75 percent sensitivity and 83 percent specificity.
To Zou’s mind, questions remain. He plans to request more samples from living patients, particularly people who are at earlier stages of their disease, to find out if the assay can detect prodromal or preclinical PD. He also will study which areas of the body provide the most accurate results. In preliminary data from two PD patients, he found that skin samples from the leg contained few seeds.—Madolyn Bowman Rogers
References
News Citations
- New Prion Detection Method
- Methods to Detect Amyloid Seeds Improve, Extend to Blood and Parkinson’s
- Test Uses 'Seeding' to Detect Aβ Oligomers in Cerebrospinal Fluid
- Spitting, Sniffing: Is This How We Will Dx Parkinson’s?
- Neurodegenerative Proteins in the Skin Could be Diagnostic
Paper Citations
- Orrú CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, Caughey B. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio. 2011;2(3):e00078-11. Print 2011 PubMed.
- Donadio V, Incensi A, Rizzo G, Capellari S, Pantieri R, Stanzani Maserati M, Devigili G, Eleopra R, Defazio G, Montini F, Baruzzi A, Liguori R. A new potential biomarker for dementia with Lewy bodies: Skin nerve α-synuclein deposits. Neurology. 2017 Jul 25;89(4):318-326. Epub 2017 Jun 30 PubMed.
- Rodriguez-Leyva I, Chi-Ahumada E, Mejía M, Castanedo-Cazares JP, Eng W, Saikaly SK, Carrizales J, Levine TD, Norman RA, Jimenez-Capdeville ME. The Presence of Alpha-Synuclein in Skin from Melanoma and Patients with Parkinson's Disease. Mov Disord Clin Pract. 2017 Sep-Oct;4(5):724-732. Epub 2017 Jun 1 PubMed.
Further Reading
News
- Can Researchers Detect Dementia With Lewy Bodies at the Prodromal Stage?
- Does Serotonin Pathology Foreshadow Parkinson’s Disease?
- Thinning Retina … Could It Be Parkinson’s?
- Touchdown for NfL: Blood Test Tells Parkinson's from Related Disorders
- Biomarkers Differentiate Parkinsonian Diseases and Forecast Decline
- Prodromal Initiative to Identify Biomarkers for Parkinson’s
Primary Papers
- Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, Incensi A, Kuzkina A, Orrú CD, Tatsuoka C, Liguori R, Gunzler SA, Caughey B, Jimenez-Capdeville ME, Zhu X, Doppler K, Cui L, Chen SG, Ma J, Zou WQ. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2020 Sep 28; PubMed.
- Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A, Adler CH, Beach TG, Kanthasamy AG. Blinded RT-QuIC Analysis of α-Synuclein Biomarker in Skin Tissue From Parkinson's Disease Patients. Mov Disord. 2020 Dec;35(12):2230-2239. Epub 2020 Sep 22 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.