Test Uses 'Seeding' to Detect Aβ Oligomers in Cerebrospinal Fluid
Quick Links
Small clusters of misfolded Aβ, called oligomers, recruit normal Aβ peptides to contort and clump up in a process called “seeding.” Some scientists hypothesize that these oligomers are the most toxic Aβ species in Alzheimer’s disease and could be a biomarker. However, oligomers are rare in cerebrospinal fluid (CSF), and scientists are still working out how to detect them (see Feb 2014 news story). In the April 10 Cell Reports, researchers led by Claudio Soto, University of Texas Medical School at Houston, report on their latest effort. They developed an assay that takes advantage of oligomers’ ability to act as seeds and distinguished people with AD from those with other neurological disorders. “Because the formation of these oligomers seems to be an early event in the disease, we may be able to use such a test for early detection,” said Soto.
Soto’s group originally developed their protein-misfolding cyclic-amplification assay (PMCA) to detect infectious prions, which cause illnesses such as Creutzfeldt-Jakob disease (CJD) (see Jun 2001 news story). It took several years for the technique to be independently reproduced, but since then, several groups have used it to study prion replication and species barriers, detect them in animals, and screen for inhibitors (Barria et al., 2012). Soto’s lab uses PMCA to detect prions in blood and urine of infected animals (see Castilla et al., 2005, and Gonzalez-Romero et al., 2008), and Soto founded a company around PMCA. A related version called real-time quaking-induced conversion, developed in Byron Caughey’s lab at the National Institutes of Health, Hamilton, Montana, is being used by some veterinary medicine labs. It can detect prions in the blood and urine of animals, including deer and elk, even before symptoms emerge, and has been used to confirm a CJD diagnosis in humans (see John et al., 2013; Orru et al., 2011; McGuire et al., 2012).
Like Aβ, pathogenic prions cause normal prion proteins to misfold and aggregate. In PMCA, a sample of a body fluid is incubated with an excess of prion protein. If the sample contains oligomers, they will convert a large number of the ordinary protein into aggregates. This process is naturally slow, so researchers speed it up by incubating the fluid sample to let oligomers grow, then breaking them up so that each smaller piece acts as a seed. This essentially amplifies prions, Soto said.
When the technique was introduced for prion protein, many scientists wondered whether it could be used to detect Aβ oligomers as well, given that they also act as seeds. However, the assay proved tricky for Aβ, said Henrik Zetterberg, University of Gothenburg, Sweden, who was not involved in the study. In his group’s hands, as well as in other labs (see Ikeda et al., 2010), CSF proved to be a potent inhibitor of Aβ aggregation. Zetterberg was surprised to see that Soto and colleagues got the assay to work for CSF Aβ.
First author Natalia Salvadores and colleagues started off testing whether their assay could pick up Aβ oligomers by making synthetic Aβ oligomers in a test tube. They incubated these with Aβ42 monomers and detected aggregating fibrils with the amyloid-binding dye Thioflavin T (ThT), which emits a quantifiable fluorescent signal. As with PMCA for prions, the researchers alternated incubation periods with shaking to break up the oligomers and speed their amplification. If just 3 fmol of oligomers were present in the original sample, the assay detected it, the researchers report.
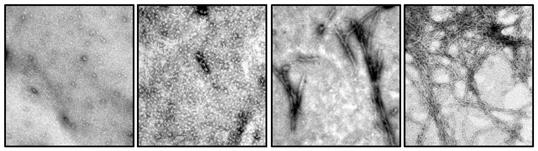
With shaking and incubation, Aβ42 monomers in a test tube (left) form oligomers (middle left), which aggregate into protofibrils (middle right) and then fibrils (right), as shown by electron microscopy. [Image courtesy of Cell Reports, Salvadores et al., Figure 1a.]
To see if the test would work with CSF, the researchers performed the same steps using patient CSF samples instead of synthetic oligomers. Samples came from 50 patients with AD, 37 with another neurodegenerative disease, and 39 cognitively normal people with a non-degenerative neurologic disease. The group estimated the lag phase, defined as the time it took the ThT signal to reach a preset value, as well as the total signal strength at 90 hours, a value they called P90. In the AD samples, the lag phase was significantly shorter, and P90 higher, than for the other two groups, suggesting aggregation occurred faster in their CSF. In samples from the two control groups, fibrils did form, but with a longer lag phase and a lower P90. The lag phase values distinguished AD from control samples with about 90 percent sensitivity and 92 percent specificity in this study.
The current study is meant as a proof of concept, Soto told Alzforum, adding that it needs further development before it can estimate the number of oligomers originally present in a sample. The authors note in the paper that monomer concentration, temperature, and pH of the buffer must be carefully controlled to get reproducible results. Since this test is not more sensitive or specific than existing CSF tests for AD, he is looking to move away from CSF and to more-accessible body fluids, such as blood. However, blood is a more complex fluid and scientists are not sure it contains oligomers, he said. Soto will also look to see if the technique picks up oligomers in people with mild cognitive impairment due to AD or prodromal disease, and asymptomatic carriers of a familial AD gene.
Scientists interviewed for this article were cautiously optimistic about the paper’s implications. “This assay really pinpoints what may be one of the basic pathogenic mechanisms in AD,” said Zetterberg. “Such an assay would be good to have.” He cautioned that getting reproducible results between labs would be a challenge because Aβ aggregation is influenced by so many factors. “The level of standardization has to be rigorous,” he said. Among other things, scientists would have to be sure that the company from which they buy Aβ peptides purified them in exactly the same way, and kept track of their oxidation level and truncation pattern.
While PMCA proved important for prion diseases, Dominic Walsh, Brigham and Women's Hospital, Boston, is unsure that it will be important for AD because there remains some doubt if Aβ oligomers determine disease to the extent prions do. Oligomer assays can address this question.
At the same time, Walsh was curious to know how the Aβ oligomer levels match up with measures of CSF Aβ and tau, and whether oligomers could add to the information these tests offer. He also wondered how oligomer levels vary between different stages of disease. If oligomers respond more dynamically to therapies than Aβ and tau, then this type of test could prove valuable, he said.—Gwyneth Dickey Zakaib
References
News Citations
- Test Closes in on Oligomers, May Distinguish Alzheimer’s Patients From Controls
- New Prion Detection Method
Paper Citations
- Barria MA, Gonzalez-Romero D, Soto C. Cyclic amplification of prion protein misfolding. Methods Mol Biol. 2012;849:199-212. PubMed.
- Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005 Sep;11(9):982-5. PubMed.
- Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008 Sep 22;582(21-22):3161-6. Epub 2008 Aug 13 PubMed.
- Orrú CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, Caughey B. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio. 2011;2(3):e00078-11. Print 2011 PubMed.
- Ikeda T, Ono K, Elashoff D, Condron MM, Noguchi-Shinohara M, Yoshita M, Teplow DB, Yamada M. Cerebrospinal Fluid from Alzheimer's disease patients promotes amyloid beta-protein oligomerization. J Alzheimers Dis. 2010;21(1):81-6. PubMed.
External Citations
Further Reading
Papers
- Santos AN, Ewers M, Minthon L, Simm A, Silber RE, Blennow K, Prvulovic D, Hansson O, Hampel H. Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer's disease. J Alzheimers Dis. 2012;29(1):171-6. PubMed.
- Yang T, Hong S, O'Malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF. Alzheimers Dement. 2013 Mar;9(2):99-112. PubMed.
- Wang-Dietrich L, Funke SA, Kühbach K, Wang K, Besmehn A, Willbold S, Cinar Y, Bannach O, Birkmann E, Willbold D. The Amyloid-β Oligomer Count in Cerebrospinal Fluid is a Biomarker for Alzheimer's Disease. J Alzheimers Dis. 2013 Jan 1;34(4):985-94. PubMed.
- Bruggink KA, Jongbloed W, Biemans EA, Veerhuis R, Claassen JA, Kuiperij HB, Verbeek MM. Amyloid-β oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal Biochem. 2012 Sep 26;433(2):112-120. PubMed.
- Santos AN, Ewers M, Minthon L, Simm A, Silber RE, Blennow K, Prvulovic D, Hansson O, Hampel H. Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer's disease. J Alzheimers Dis. 2012;29(1):171-6. PubMed.
Primary Papers
- Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer's disease. Cell Rep. 2014 Apr 10;7(1):261-8. Epub 2014 Mar 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.