Spitting, Sniffing: Is This How We Will Dx Parkinson’s?
Quick Links
The quest for a noninvasive diagnostic test is leading Parkinson’s scientists to unlikely places. One is inside the mouth. At the 14th International Conference on Alzheimer’s and Parkinson’s Diseases in Lisbon, Portugal, Giorgio Vivacqua, University of Cambridge, U.K., described his search for pathological α-synuclein in saliva. He claimed that an assay that quantifies synuclein species capable of seeding aggregation was able to pick out PD with high accuracy in a small pilot trial. Another is the spot on the back between a person’s shoulder blades. There, researchers are analyzing a distinctive odor reported to cling to people with PD. According to work from the lab of Perdita Barran, a mass spectrometrist at the University of Manchester, U.K., the musty scent arises from compounds that accumulate in sebum, the waxy, lipid-packed secretion of the skin’s sebaceous glands. A mixture of four volatile chemicals found in sebum largely replicated the “PD smell.” Quantifying their concentration allowed the researchers to distinguish healthy people from those with PD. Alas, for both spit and skin tests, much more research lies between these initial results and a certified, widely available test.
Walter Koroshetz, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, called the saliva test impressive. “These are still small numbers, but they show good specificity,” he told Alzforum.
Tiago Outeiro, who has university appointments in Göttingen, Germany, and Lisbon, called the sebum work exciting. “It will require a lot of additional work to demonstrate whether this has diagnostic or predictive potential, but we need to keep an open mind about new approaches and follow this up, because it may help us diagnose disease in its early stages,” he told Alzforum.
In a plenary talk at AD/PD, Brit Mollenhauer of Paracelsus-Elena-Klinik, Kassel, Germany, showed examples of how the current, largely clinical diagnosis of Parkinsonian disorders can lead to misdiagnoses that worsen patient care and outcomes. She called on more researchers in the field to join the search for biomarkers at the pre-motor stage of those diseases. Much of that work in the PD field focuses on CSF and blood, REM sleep disorder, and unspecific features such as loss of the ability to distinguish odors. Emerging biomarker studies in saliva and sebum are less well known, and as yet the large cohorts being built to search for biomarkers do not all collect saliva and sebum needed to replicate their early work in larger samples.
Biomarkers for the hallmark pathology of PD—aggregates of misfolded α-synuclein—would be invaluable for definitive diagnosis, to track disease progression, and to evaluate the effectiveness of synuclein-targeted therapies.
Despite initial promise, CSF measures of total α-synuclein have not caught on for diagnosing or tracking progression. People with PD have on average lower CSF α-synuclein, but there’s a large overlap between healthy and disease groups, and new data from the longitudinal Parkinson’s Progression Marker Initiative (PPMI) found little change with time. A new tack may be to zero in on misfolded α-synuclein leaching from the brain’s extracellular space into the CSF. Misshapen or aggregated species have been difficult to reliably measure with ELISA, but that is changing with seeded aggregation assays.
Originally developed to detect infectious prions, these assays, aka protein misfolding cyclic amplification (PMCA) or real‐time quaking‐induced conversion (RT-QuIC), rely on the ability of pathological α-synuclein to induce misfolding of native α-synuclein. In seeded aggregation, a minute amount of CSF sample is incubated with a large excess of recombinant α-synuclein. The mixture is put on a shaker for some hours and, in time, misfolded α-synuclein in the sample initiates condensation of the normal protein into amyloid fibrils, which are quantified by monitoring binding of the fluorescent dye Thioflavin T (ThT). Applied to CSF, RT-QuIC for α-syn seeds can distinguish healthy people from those with Parkinson’s disease with greater than 90 percent sensitivity and specificity (Dec 2016 news; Fairfoul et al., 2016; Groveman et al., 2018).
Perhaps surprisingly, saliva, which is easier to collect than CSF, appears to harbor misfolded synuclein, as well, as Vivacqua and others discovered using Western blotting and ELISA (Vivacqua et al., 2016; Kang et al., 2016; Vivacqua et al., 2019). The likely sources are the autonomic nerves that innervate the salivary glands, which in people with PD are dotted with α-syn aggregates (Beach et al., 2010). Currently, biopsies of the submandibular gland (SMG) and skin provide the only way to document α-synuclein pathology in living people, albeit outside the brain. Some of the large PD biomarker cohorts do include biopsy substudies. Alas, while SMG biopsy carries fewer risks than lumbar puncture, it is uncomfortable, generates usable tissue only 65 percent of the time, and requires the expertise of an ear, nose, and throat specialist (Chahine et al., 2018).
Working in Maria-Grazia Spillantini’s lab, Vivacqua applied RT-QuIC analysis to saliva collected from 36 people who had had PD for an average of two years, and 23 age- and sex-matched healthy volunteers. For quantitation, the researchers tallied the lag time before the rise in fluorescence began, the speed of the increase over 60 hours, and the percent increase at 60 hours. They tested each patient sample three times, in triplicate each time, and achieved high inter-replicate and test-retest reproducibility, Vivacqua said.
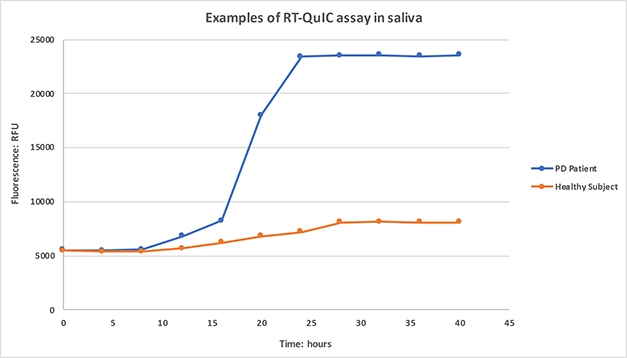
Spitting Seeds. Saliva from PD patients, but not healthy controls, initiates α-synuclein aggregation, as detected by increasing ThioflavinT fluorescence. [Courtesy of Giorgio Vivacqua.]
Samples that failed to boost fluorescence above a set threshold after 60 hours were deemed negative. Among the healthy controls, 74 percent (17 of 23) were negative, though 30 percent of the PD patients, or 11 of 36, apparently were as well. Three of these negative patients went on to be diagnosed with tauopathies—two with frontotemporal dementia and one with progressive supranuclear palsy—suggesting they initially had been misdiagnosed with PD.
The as-yet unexplained mismatches in Vivacqua’s study should be followed up, Koroshetz told Alzforum. It would be interesting to know if saliva results correlate with presence of synuclein in submandibular glands, which would give ground truth to the RT-QuIC test, and also to know if the saliva aggregation-positive healthy volunteers become symptomatic, whether with parkinsonism or REM sleep disorder, he said.
For aggregation-positive samples, the lag phase was shorter and fluorescence rose faster in cases compared to controls. These measures distinguished healthy from PD with a sensitivity of 79 and 83 percent, respectively, and a specificity of 100 percent, Vivacqua showed. The fluorescence increase, which could be quantitated in both positive and negative samples, was larger in PD patients, but gave a less-precise separation of the groups, with only 60 percent sensitivity and 88 percent specificity. Vivacqua said he had not yet studied how the RT-QuIC results of α-synuclein in saliva correlate with clinical measures. “We think this is promising for molecular diagnosis of PD,” he concluded.
Michael Schlossmacher, Ottawa Hospital Research Institute, Canada, agreed that saliva is an attractive source of PD biofluid for analysis, but cautioned it may pose unique challenges compared with CSF. Saliva production and concentration fluctuate over the course of the day. Spit harbors a host of microbes, as well as digestive enzymes that chew up carbohydrates and some proteins. “The assays need more subjects and proper consideration of these variables, including medication details. On the plus side, the ease of collection and the RT-QuIC assay lends itself to rapid study of more cohorts,” Schlossmacher said.
Whether spit will signal PD before symptoms begin remains to be seen. Thomas Beach, Banner Sun Health Research Institute, Sun City, Arizona, said he finds Lewy-type α-synuclein deposits in submandibular glands at autopsy in only a third of people who die with asymptomatic α-synuclein brain pathology. Perhaps doing RT-QuIC on SMG or skin biopsy tissue would yield more positives, he said.
Saliva may yet prove to be a good marker of progression. At AD/PD, Beach showed data that serial SMG biopsies revealed a fourfold worsening of α-syn aggregates over four years. It will be interesting to see if that is reflected in saliva, Beach told Alzforum.
Vivacqua said that one outstanding challenge is to characterize the seeds in saliva. Could other aggregated proteins, such as tau, also be present there, and interfere with the assay? “We need to address this, because it is possible that other clinical forms of Parkinsonism could have different types of seed in saliva, as they do in CSF,” he said.
Claudio Soto, University of Texas Medical School at Houston, developed the PMCA assay for prions but was not involved in Vivacqua’s study. He told Alzforum that the Cambridge group has made a good start. To commercialize this assay, Soto founded a company, which he said has developed a faster, automated version that will be used to measure seeding in CSF samples from PPMI. Soto’s current focus is developing the assay for blood, but his group is also investigating saliva, tears, and urine, and even extracts of skin.
The Nose Knows
Other groups are also chasing clues coming off the skin. According to a report published March 20 in ACS Central Science, co-author Joy Milne is a super smeller who can distinguish scents better than most people. Her husband, Les, was diagnosed with PD at age 45 in 1986. A nurse, Milne reported that six years earlier, he had developed a musky odor, which disappeared with treatment and returned as his disease advanced (Morgan, 2016). Scientists began to investigate her story and indeed, tested her nose on T-shirts worn by people with and without PD. She could pick out the disease with perfect accuracy.
The odor Milne picked out was not in sweat. It was in sebum, the light yellow, oily secretion of the sebaceous glands that moisturize skin and hair. Excessive sebum secretion is a non-motor symptom of PD, and likely due to changes in the sympathetic nerves that innervate the glands.
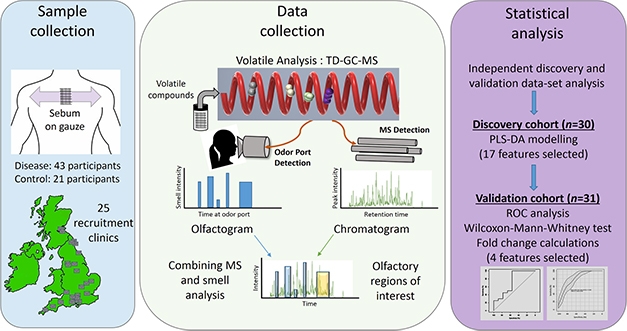
Follow Your Nose. Study scheme to identify volatile compounds responsible for distinctive odor associated with PD. [Courtesy of Trivedi et al., 2019.]
In the new study, first author Drupad Trivedi led the effort to identify the compounds that make up the PD odor (Trivedi et al., 2019). The team collected sebum samples by swabbing medical gauze on the upper backs of patients and matched controls, bagging the gauze, and sending it to a central mass spec lab at the University of Manchester. There, scientists heated the sebum samples to release volatile organic compounds, which were separated and identified by gas chromatography (GC)-mass spec.
The investigators used a discovery cohort of 30 cases and controls, and a separate validation cohort of similar size, recruited from 25 NHS clinics across the U.K. They identified four chemicals in the sebum volatilome whose levels told PD from controls with 90 percent accuracy. Three of the compounds, hippuric acid, eicosane, and octadecanal, were all elevated in patients, although only eicosane levels were statistically significant. In some experiments, the chromatography products went to an odor-detection port, where Milne was stationed to smell the separated compounds. She confirmed the PD odor in the chromatography eluate containing the three compounds. The fourth, perillic aldehyde, was significantly lower in PD sebum. A cocktail of the four chemicals, spiked into normal sebum, mimicked the PD smell closely, according to Milne.
“This is a really well-done study,” said Schlossmacher, who is not a part of this research. “It’s intriguing that both eicosane and perillic aldehyde have also been identified in normal human saliva, as the authors note. Some practitioners, including myself, sense that the ‘PD odor’ in late-onset cases may be associated with changes in our patient’s breath, as well.”
Researchers are pursuing volatilome analysis of breath to detect pulmonary disease and lung cancer (Chang et al., 2018; Lawal et al., 2018), but Schlossmacher said he did not know if this had been done for PD yet.
The published work is part of the larger Nose2Diagnose study led by Barran, which began in 2017. Trivedi told Alzforum the Manchester group is scaling up to validate the results in an additional 350 sebum samples. Ultimately, they will collect 2,000 samples to look at various markers from PD sebum, he said.
Human super smellers are rare, but there is a species whose famously fine nose has stood in the service of humanity for centuries. According to press reports, the researchers are working with a medical dog company to train canines to sniff out PD.—Pat McCaffrey and Gabrielle Strobel
References
News Citations
Paper Citations
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Baig F, Ruffmann C, Wade-Martins R, Hu MT, Parkkinen L, Green AJ. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016 Oct;3(10):812-818. Epub 2016 Aug 28 PubMed.
- Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, Campbell KJ, Safar J, Galasko D, Caughey B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018 Feb 9;6(1):7. PubMed. Correction.
- Vivacqua G, Latorre A, Suppa A, Nardi M, Pietracupa S, Mancinelli R, Fabbrini G, Colosimo C, Gaudio E, Berardelli A. Abnormal Salivary Total and Oligomeric Alpha-Synuclein in Parkinson's Disease. PLoS One. 2016;11(3):e0151156. Epub 2016 Mar 24 PubMed.
- Kang W, Chen W, Yang Q, Zhang L, Zhang L, Wang X, Dong F, Zhao Y, Chen S, Quinn TJ, Zhang J, Chen S, Liu J. Salivary total α-synuclein, oligomeric α-synuclein and SNCA variants in Parkinson's disease patients. Sci Rep. 2016 Jun 23;6:28143. PubMed.
- Vivacqua G, Suppa A, Mancinelli R, Belvisi D, Fabbrini A, Costanzo M, Formica A, Onori P, Fabbrini G, Berardelli A. Salivary alpha-synuclein in the diagnosis of Parkinson's disease and Progressive Supranuclear Palsy. Parkinsonism Relat Disord. 2019 Jun;63:143-148. Epub 2019 Feb 14 PubMed.
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG, . Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010 Jun;119(6):689-702. PubMed.
- Chahine LM, Beach TG, Seedorff N, Caspell-Garcia C, Coffey CS, Brumm M, Adler CH, Serrano GE, Linder C, Mosovsky S, Foroud T, Riss H, Ecklund D, Seibyl J, Jennings D, Arnedo V, Riley L, Dave KD, Mollenhauer B, Systemic sSynuclein Sampling study. Feasibility and Safety of Multicenter Tissue and Biofluid Sampling for α-Synuclein in Parkinson's Disease: The Systemic Synuclein Sampling Study (S4). J Parkinsons Dis. 2018;8(4):517-527. PubMed.
- Morgan J. Joy of super smeller: sebum clues for PD diagnostics. Lancet Neurol. 2016 Feb;15(2):138-139. Epub 2016 Jan 12 PubMed.
- Trivedi DK, Sinclair E, Xu Y, Sarkar D, Walton-Doyle C, Liscio C, Banks P, Milne J, Silverdale M, Kunath T, Goodacre R, Barran P. Discovery of Volatile Biomarkers of Parkinson's Disease from Sebum. ACS Cent Sci. 2019 Apr 24;5(4):599-606. Epub 2019 Mar 20 PubMed.
- Chang JE, Lee DS, Ban SW, Oh J, Jung MY, Kim SH, Park SJ, Persaud K, Jheon S. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sensors and Actuators B: Chemical February 2018
- Lawal O, Muhamadali H, Ahmed WM, White IR, Nijsen TM, Goodacre R, Fowler SJ. Headspace volatile organic compounds from bacteria implicated in ventilator-associated pneumonia analysed by TD-GC/MS. J Breath Res. 2018 Jan 3;12(2):026002. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Trivedi DK, Sinclair E, Xu Y, Sarkar D, Walton-Doyle C, Liscio C, Banks P, Milne J, Silverdale M, Kunath T, Goodacre R, Barran P. Discovery of Volatile Biomarkers of Parkinson's Disease from Sebum. ACS Cent Sci. 2019 Apr 24;5(4):599-606. Epub 2019 Mar 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Ottawa Hospital Research Institute
Trivedi et al. is a really well-done pilot study that builds on a comprehensive volatilome-analyzing platform.
I find it intriguing that both eicosane and perillic aldehyde have also been identified in saliva, as the authors note. Some practitioners (including myself) sense that a “PD subject’s odor” in late-onset cases may be associated with changes in their breath, as well. I do not know whether this has been analyzed yet.
In the future it will be important to attempt to control for many variables in a comparison of PD versus controls, e.g. medication, age, sex, perfume/deodorant use, diet, hydration status, bathing habits, clothing fabric, exercise, etc.
Personally, I see two angles to further investigate, i.e., the skin microbiome as well as skin neurophysiology. For the former, there is an exciting rosacea story from the Danish registry, showing rosacea is a skin disease that poses a modifiable risk factor for PD (Egeberg et al., 2016). For the latter, there is published work by the Freeman lab on α-synucein staining of skin biopsies (Gibbons et al., 2016), which the team of Dr. Wolfgang Oertel in Germany is actively pursuing, as well.…More
References:
Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Exploring the Association Between Rosacea and Parkinson Disease: A Danish Nationwide Cohort Study. JAMA Neurol. 2016 May 1;73(5):529-34. PubMed.
Make a Comment
To make a comment you must login or register.