It Bleeds! New Mini-Brains Sport Functioning Blood Vessels
Quick Links
While scientists can grow brain-like organoids in a culture dish, these tiny structures still lack many of the features of the real brain. Crucially, they don’t form a vascular system, which is essential for delivering oxygen to neurons deep within the structure. Now, In-Hyun Park at Yale School of Medicine in New Haven, Connecticut, and colleagues have generated cortical organoids that appear to have a functioning vasculature. In the October 7 Nature Methods, they describe how a single transcription factor coaxed some of the starting stem cells that go on to build organoids to form tubes lined with endothelial cells. These organoids had healthier, more mature neurons than did organoids without blood vessels. The vessels transported fluid and formed the tight junctions between endothelial cells typical of the blood-brain barrier. Aβ42 oligomers disrupted these junctions, allowing molecules to leak into the neural tissue. Vascularized organoids could provide a model for investigating brain development and disease mechanisms, the authors believe.
- New protocol generates cortical organoids with blood vessels.
- Cells lining these vessels form tight junctions, as in the blood-brain barrier.
- Neurons in these organoids stay healthy for months.
Other researchers agreed, calling the model an important advance. “This paper introduces a new approach that improves upon and extends the capabilities of cortical organoids, and promises to facilitate deeper investigation into the complex biology underlying Alzheimer’s disease,” Li-Huei Tsai and Joel Blanchard at Massachusetts Institute of Technology, Cambridge, wrote to Alzforum (full comment below). Weiming Xia at Boston University noted that the vascularized organoids could be used for high-throughput drug screening. “This system … has tremendous potential for testing therapeutic agents and determining their permeabilities across the blood-brain barrier,” he wrote (full comment below).
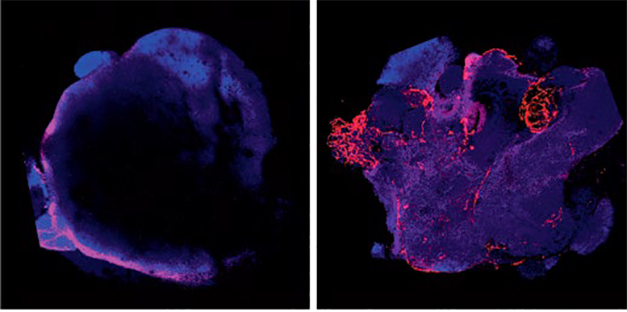
Now With Blood! By nudging stem cells toward an endothelial fate (right), researchers generated cortical organoids with a vasculature (red). Control organoids (left) express few endothelial markers. Neurons are purple. [Courtesy of Cakir et al., Nature Methods.]
Researchers generated the first cortical organoids in 2013. They seeded stem cells into a three-dimensional matrix in a nutrient bath. Over a month, the cells differentiated and formed small, brain-like structures a few millimeters wide. These possessed cell layers, and they mimicked structures such as the hippocampus and cerebral cortex (Aug 2013 news). Without a blood supply, however, neurons in the center of these organoids eventually starved and died.
Park and colleagues wondered if prodding stem cells to become endothelial cells might spur vascular development. Joint first authors Bilal Cakir and Yangfei Xiang transduced 20 percent of the starting stem cell pool with a lentivirus carrying the endothelial transcription factor ETV2, under the control of an inducible promoter. The authors turned on ETV2 expression after 18 days in culture, when organoids were already forming. By 1 month of age, the organoids had developed tube-shaped vessels that expressed endothelial cell markers. These tubes transported a labeled marker throughout the organoid, suggesting they formed a working vascular system.
The vessels expressed numerous blood-brain barrier markers, such as α-ZO1, occludin, and claudin-5. Cells around the vessels expressed markers of pericytes, tiny cells that hug capillaries in the brain and are essential for blood-brain barrier formation during embryonic development (Daneman et al., 2010). In control organoids, blood-brain barrier markers were sparse, pericyte markers absent. Electrical resistance across vessel walls was high, indicating a tight barrier between lumen and neural tissue.
To further test for blood-brain barrier function, the authors added Aβ42 to the culture media. Oligomers, but not fibrils, abolished α-ZO1 staining and caused vessels to leak labeled tracer into the surrounding tissue. Aβ has been shown to disrupt the blood-brain barrier in mice (Sep 2015 news). “Vascular cortical organoids may be a useful model for investigating how amyloid and other pathogenic protein oligomers influence BBB properties,” Tsai and Blanchard wrote. Park told Alzforum that he will add microglia to the vascularized organoids, and use the model to further investigate the effects of Aβ and other AD-related proteins.

Aβ Causes Leakage. In vascularized organoids (top), a fluorescent tracer (green) stays inside blood vessels, but after addition of Aβ42 oligomers (bottom), tracer leaks out. Nuclei are blue. [Courtesy of Cakir et al., Nature Methods.]
Vascularization Boosts Neuronal Function
Initially, vascularized organoids grew more slowly, but they caught up by two months, when control organoids had stopped growing and were about four millimeters in diameter. Neurons in vascularized organoids stayed healthy for months, with almost no apoptosis. In contrast, 40 percent of cells in control organoids had died after four months.
Neurons in vascularized organoids seemed more mature. Transcriptional profiling placed their effective age at gestational week 16–19, compared with week 10–12 for neurons in control organoids. In keeping with this, neurons in vascularized organoids were more electrically active. Almost half of them began firing bursts of action potentials after three months in culture, a time at which nearly all neurons in control organoids remained silent. “This is important, because a major barrier to using organoids for modelling neurodegenerative disease is their relative immaturity,” noted Selina Wray, Charlie Arber, and Christopher Lovejoy at University College London, in an email to Alzforum (full comment below). Previous research had suggested that endothelial cells spur neuronal birth and maturation, which may explain the activity in vascularized organoids (Jin et al., 2002; Shen et al., 2004; Paredes et al., 2018).
Researchers noted that challenges remain. Cortical organoids are notoriously variable from one to the next, making it difficult to replicate findings. A recent study reported that specific growth factors could push differentiation of developing organoids in a more uniform direction (Jun 2019 news). Whether that will work with the vascularized methodology needs to be determined. Likewise, it remains to be seen how mature the vasculature is, given that cortical organoids tend to model fetal brain. “Vasculature in adult brain is much more complex, including perivascular spaces that contribute to the clearance of toxic solutes such as Aβ,” Axel Montagne and Mikko Huuskonen at the University of Southern California, Los Angeles, wrote to Alzforum (full comment below). They suggested that future studies examine whether vessels in cortical organoids can dilate and contract and whether neuronal activity can influence this, as it does in the brain. “The next few years of research using vascularized brain organoids will provide more answers,” they noted.
Other researchers have taken different approaches to modeling the blood-brain barrier in vitro. Cheryl Wellington of the University of British Columbia in Vancouver, Canada, has generated artificial cerebral blood vessels in a reactor using tissue engineering on a tubular scaffold (Oct 2017 news; Apr 2019 conference news). Don Ingber and Kit Parker at Harvard used a series of interconnected polymer chips to model interactions between brain cells and blood vessels (Aug 2018 news).
Recently, Doo Yeon Kim, Se Hoon Choi, and Rudolph Tanzi at Harvard Medical School in Charlestown, Massachusetts, and Roger Kamm at MIT added a vascular component to their three-dimensional neural culture model (Oct 2014 news). They established the culture on a five-channel microfluidic plate, with the middle channel initially empty. On one side of it, Choi and co-first author Yoojin Shin at MIT grew a three-dimensional neuronal culture in a gel matrix. On the other side, they placed endothelial cells on a collagen scaffold. Once the cultures had matured, the authors added hydrogel to the middle channel to allow the cells to interact. The endothelial cells began expressing blood-brain barrier markers, formed tight junctions, and held back the passage of solutes to the neuronal chamber. When the cultured neurons carried AD mutations, Aβ40 and Aβ42 deposited on the endothelial cell layer, and solutes flowed more freely across it, mimicking the cerebral amyloid angiopathy and blood-brain barrier disruption seen in AD. The findings are described in the August 2019 Advanced Science.—Madolyn Bowman Rogers
References
News Citations
- Mini Brain in a Dish Models Human Development
- Barriers Between Blood and CSF, Brain Yield to Aβ—Not a Bad Thing?
- Reproducible Brain Organoids Could Offer New Models for Research
- Artificial Human Blood Vessels: A Model for Cerebral Amyloid Angiopathy?
- Could Greasing the Wheels of Lipid Processing Treat Alzheimer’s?
- 'Organ on a Chip' Models the Ins and Outs of the Blood-Brain Barrier
- Alzheimer’s in a Dish? Aβ Stokes Tau Pathology in Third Dimension
Paper Citations
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010 Nov 25;468(7323):562-6. Epub 2010 Oct 13 PubMed.
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11946-50. Epub 2002 Aug 14 PubMed.
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004 May 28;304(5675):1338-40. Epub 2004 Apr 1 PubMed.
- Paredes I, Himmels P, Ruiz de Almodóvar C. Neurovascular Communication during CNS Development. Dev Cell. 2018 Apr 9;45(1):10-32. PubMed.
- Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE. Blood-Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer's Disease. Adv Sci (Weinh). 2019 Oct 16;6(20):1900962. Epub 2019 Aug 12 PubMed.
Further Reading
News
- Absent Aβ, Blood-Brain Barrier Breakdown Predicts Cognitive Impairment
- Ring Around the Vessel: Enlarged Spaces Signal Vascular Disease
- Using Clinical Dx Only, Study Calls Stroke Clear Risk Factor for LOAD
- LOAD of Data Place Vascular Malfunction as Earliest Event in Alzheimer’s
- Microbleeds in the Brain Portend Dementia
- Does the Blood-Brain Barrier Stand Up to Alzheimer’s? Study Finds No Breach
Primary Papers
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MS, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019 Oct 7; PubMed.
- Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE. Blood-Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer's Disease. Adv Sci (Weinh). 2019 Oct 16;6(20):1900962. Epub 2019 Aug 12 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Picower Institute of MIT
Icahn School of Medicine at Mt. Sinai
Cortical organoids offer powerful technology for modeling brain development and other neurological disorders. However, cortical organoids have a high degree of inter-organoid variability, lack microglia and vascular cells, and often contain necrotic regions due to limited oxygen and nutrient diffusion (Kelava and Lancaster, 2016). Cakir and colleagues find that overexpressing ETV2 in a population of cells in developing cortical organoids leads to induced vascular structures within the organoid and a dramatic reduction of cell death. Intriguingly, using single-cell RNA-Seq they also find the presence of PDGFRβ-positive cells suggesting the presence of pericytes, which play a key role in the formation of the blood-brain barrier (Armulik et al., 2010; Daneman et al., 2010). Cakir and colleagues do find that whole organoids that are vascularized exhibit increased electrical resistance and they observe on endothelial cells the presence of the tight junction protein ZO1, both of which are consistent with barrier formation. Further studies should determine whether additional molecular, anatomical, and physiological properties of the blood-brain barrier (BBB) are present.
In particular, pericytes and the BBB are increasingly recognized to play an important role in Alzheimer’s disease and neurodegeneration (Halliday et al., 2015; Nation et al., 2019; Nortley et al., 2019; Sagare et al., 2013). However, there are limited human model systems for investigating pericyte biology in multicellular environments. Therefore, it will be important to determine the extent to which the pericytes in these vascular organoids reflect human brain pericytes and whether they are properly localized on organoid microvasculature. Cakir and colleagues also demonstrate that the induced vasculature of organoids exhibit a decrease in the ZO1 tight junction protein and increased permeability in response to Aβ1-42 oligomers. This suggests that these vascular cortical organoids may also be a useful model for investigating how amyloid and other pathogenic protein oligomers influence BBB properties. In particular, future studies may apply this technology to examine how amyloid alters BBB phenotypes between isogenic pairs of iPSCs containing genetic risk factors associated with AD.
Ultimately, this paper introduces a new approach that improves upon and extends the capabilities of cortical organoids and promises to facilitate deeper investigation into the complex biology underlying AD.
References:
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010 Nov 25;468(7323):557-61. Epub 2010 Oct 13 PubMed.
Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010 Nov 25;468(7323):562-6. Epub 2010 Oct 13 PubMed.
Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab. 2015 Mar 11; PubMed.
Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TL, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019 Feb;25(2):270-276. Epub 2019 Jan 14 PubMed.
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D. Amyloid β oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science. 2019 Jul 19;365(6450) Epub 2019 Jun 20 PubMed.
Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. PubMed. Correction. RETRACTED
Kelava I, Lancaster MA. Stem Cell Models of Human Brain Development. Cell Stem Cell. 2016 Jun 2;18(6):736-48. PubMed.
University of Edinburgh
University of Southern California
This article by Cakir et al. provides what we believe to be a very important method for modeling a human brain in a dish with a functional vascular-like network. In this paper, Dr. Park and his colleagues demonstrated that it is possible to obtain vascularized human cortical organoids (vhCOs) out of human ETS variant 2 (ETV2)-induced reprogramming of human embryonic stem cells (hESCs). The authors convincingly proved that vhCOs possess classical blood-brain barrier (BBB) properties—i.e., expression of tight junctions (ZO-1, Occludin, and Claudin-5) and nutrient transporters (GLUT1 and P-glycoprotein), as well as high trans-endothelial electrical resistance (TEER). Importantly, the single-cell transcriptomic analysis not only revealed the presence of endothelial- and pericyte-specific markers but also demonstrated that organoid vascularization promotes neuronal maturation. In a last set of experiments, the authors established that vhCOs can form functional blood vessels in vivo when implanted subcutaneously into the hindlimbs of immune-deficient mice.
Although this is a crucial step forward for the vascular field, there are number of questions and many more challenges that still lie ahead in applying current brain organoid technology to study the vascular contribution to neurodegenerative diseases. First of all, future studies would have to tackle the fundamental question of reproducibility and functional homogeneity given the known organoid-to-organoid variability. Also, the current method is using hESCs technology modeling fetal brain-like organoids, which may be somewhat limited in recapitulating a mature brain environment. One should keep in mind that this is a developmental model, vasculature in adult brain is much more complex including perivascular spaces, for instance, that contribute to the clearance of toxic solutes such as Aβ. In a context of Alzheimer’s disease, it is not known thus far whether the two-hit vascular hypothesis—that cerebrovascular damage (hit 1) is an initial insult that is self-sufficient to initiate neurodegeneration, but can also promote accumulation of Alzheimer's Aβ toxin in the brain (hit 2)—can be tested in this model.
Finally, one may think that moving toward inducible pluripotent stem cells (iPSCs) might be beneficial to study unique mutations and single-nucleotide polymorphisms and test their effects on vascular and neuronal functions in a context of a specific neurodegenerative disease. It will be very interesting to now investigate whether the blood vessels are able to constrict and dilate, whether pericytes can contract and relax, whether vhCOs would be useful to study neurovascular coupling, whether a venous and arterial flow exist, and many more. The next few years of research using vascularized brain organoids will provide more answers and undoubtedly a breakthrough in the cerebrovascular field.
Institute of Neurology, University College London
UCL Queen Square Institute of Neurology
UCL
Since the milestone paper in 2013 by Lancaster et al., cerebral organoids have provided a means to generate complex, three-dimensional, in vitro models of human neurons recapitulating, in part, neuronal diversity and aspects of the physical architecture of the developing brain, such as lamination. Due to the differing development ontogenies of specific cell types, cerebral organoids cannot fully capture cellular diversity through intrinsic differentiation alone, and the absence of microglia and vasculature is particularly relevant in the context of AD. The absence of vasculature results in organoids becoming size-limited, and having a largely necrotic core due to the inability of media to penetrate into the center of the structure.
Previous work from Mansour et al. (2018) showed that organoids transplanted into mice developed vasculature in vivo. In this exciting new paper, Cakir et al. describe a new method for in vitro vascularization by engineering a subset of hESC to ectopically express ETV2, which overrides the intrinsic signaling of cells during organoid differentiation to drive patterning into endothelial cells (ECs) which form complex, vasculature-like structures within the organoids (vascularized organoids, vhCOs). They convincingly show, using multiple methods (MRI, gadolinium, and FITC) this leads to a perfusable, vascularized organoid in vitro, and in vivo following transplantation. Interestingly, vhCOs show accelerated electrophysiological maturation, a finding supported by scRNA-Seq data which demonstrate that vhCOs have a transcriptomic signature that is representative of a more mature developmental stage than unvascularized COs. This is important because a major barrier to using organoids for modelling neurodegenerative disease is their relative immaturity, as demonstrated by transcriptomic signatures akin to fetal neurons (Lindahl, 1989) as well as oscillatory waves similar to synchronous networks observed in preterm fetal brain (Trujillo et al., 2019). Methods that enhance the health, maturity, and function of neurons within organoids will be welcomed by the field.
These, together with recent reports describing the incorporation of iPSC-microglia into organoids (Abud et al., 2017), pave the way to achieve complex, multicellular, human in vitro models that permit the study of non-cell autonomous disease mechanisms in AD and provide human models of the blood brain barrier—a key tool for testing drug delivery to the brain.
References:
Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018 Jun;36(5):432-441. Epub 2018 Apr 16 PubMed.
Lindahl G. Cell surface proteins of a group A streptococcus type M4: the IgA receptor and a receptor related to M proteins are coded for by closely linked genes. Mol Gen Genet. 1989 Apr;216(2-3):372-9. PubMed.
Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell. 2019 Oct 3;25(4):558-569.e7. Epub 2019 Aug 29 PubMed.
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
Boston University School of Medicine
Cakir et al. have established a brain organoid model carrying a vascular-like system that functions in implanted mice. This system is unique in many ways compared with previous published brain organoids, and it has tremendous potential for testing therapeutic agents and determining their permeabilities across the blood brain barrier (BBB).
While standard in vitro permeability assays serve as the starting point to eliminate many compounds with poor brain-to-plasma ratios, a more physiologically relevant model mimicking brain environment is desirable. For example, Cakir et al. demonstrated damage to vascular structures by oligomeric Aβ42 species (not Aβ fibrils) in their brain organoids. An interesting question is, is it possible that high levels of oligomeric Aβ comprise cerebrovascular integrity and allow therapeutic biologics and small molecule compounds to freely pass through the BBB in brains of Alzheimer's disease patients? On the other hand, ApoE is known to maintain BBB function, but ApoE-4 acts in an opposite way. With all genetic tool sets, those variables can be incorporated into this reported brain organoid system to model aging brains in AD patients.
An important application of brain organoids is to create an in vitro model for high throughput drug screening. The reported model system could be used to test permeability of a large molecular reporter, such as FITC-dextran, as well as small molecule therapeutics. Matrix-assisted laser desorption/ionization-mass spectrometry imaging could be applied to visualize the distribution of compounds and semi-quantify the drug exposure within the organoids, as previously reported (Cho et al., 2017). This approach is similar to the detection of FITC-dextran under a fluorescent microscope. More importantly, biochemical readouts can be obtained from adjacent sections of brain organoids, and efficacy of compounds can be tested. Thus, the reported brain organoid system with vascular structure may provide spatial resolution in addition to the classic drug exposure-efficacy relationship for compounds of interest.
References:
Cho CF, Wolfe JM, Fadzen CM, Calligaris D, Hornburg K, Chiocca EA, Agar NY, Pentelute BL, Lawler SE. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun. 2017 Jun 6;8:15623. PubMed.
Massachusetts General Hospital
Massachusetts General Hospital
Massachusetts General Hospital
This study is a step forward to a more physiologically relevant brain organoid model. Dr. Park’s team elegantly showed a vascularized human cortical organoid (vhCO), which displays blood-brain-barrier (BBB) characteristics and vasculature-like networks in vitro and in vivo. In addition to basic studies, this new vhCO model has the potential to accelerate CNS drug testing by providing a BBB-like permeability barrier, which has not been feasible with current brain organoid models. Further studies will be needed to test if this new vhCO model can achieve enough organoid-to-organoid structural homogeneity for large-scale drug testing.
Regarding Alzheimer’s disease, the authors showed that oligomeric synthetic Aβ preparations selectively disrupted BBB permeability in their vhCOs. This result is consistent with our recent publication with an engineered human three-dimensional AD BBB model. In this paper, we showed that pathogenic Aβ species, secreted from familial AD neurons, selectively disrupted the BBB permeability and even were deposited to the surface of the engineered three-dimensional human endothelial layers. Emerging evidences strongly support roles of vasculature and BBB in AD pathogenesis on multiple levels (Deane and Zlokovic, 2007). We hope that technical advances in this study lead to development of more sophisticated three-dimensional AD brain models that can comprehensively recapitulate vascular dysfunction and its contribution to AD pathogenesis.
References:
Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007 Apr;4(2):191-7. PubMed.
University of Minnesota, Twin Cities
University of Minnesota
University of Minnesota
University of Minnesota, Twin Cities
Human brain organoids have emerged as a promising model to study neural development and neurological disorders, including Alzheimer’s disease. However, it remains challenging to generate a bona fide model that recapitulates the complex structure and function of the human brain. Cakir et al. have developed a method addressing an important component of organoid modeling, vasculature. They generated vhCOs (vascularized human cortical organoids) by ectopically expressing human ETS variant 2 to drive endothelial lineage. They found that the vhCOs developed a complex vascular-like network that is perfusable. The vhCOs were healthier, as indicated by less TUNEL staining compared to hCOs. When the authors compared single-cell transcriptomes, they found that the vhCOs expressed more mature neuronal markers, as well as markers of other cell types, in particular those involved in the formation of neurovascular units, including tight junction markers, astrocytic and pericytic proteins, and transporters. Notably, trans-endothelial electrical resistance (TEER) analysis showed that vhCOs formed tight junctions comparable to other three-dimensional BBB models. Furthermore, treating the vhCOs with oligomeric Aβ42 led to the malformation of tight junctions and the disruption of the BBB, illustrating that the BBB-like structure responds to exogenous factors. The vhCOs also had better success grafting in vivo in mice and formed functional vascular connections to the host mouse.
Making in vitro brain organoids healthier, more complex, and more physiological is a very encouraging and long-awaited development. This model, coupled with a perfusion system mimicking the blood circulation, may provide an authentic BBB model to study the interactions between the CNS and peripheral systems, such as mechanisms regulating the trafficking of Aβ or other molecules across the BBB, and to screen for drugs that modulate BBB permeability. For modeling neurological diseases, it would be good to know if this method also works for other human ESCs and iPSCs and if the pattern of vascularization is consistent between cultures and lines. In addition, the vhCOs described here lack microglial cells that play pivotal roles in the brain under physiological and pathological conditions. It will be interesting to see how the presence of those immune cells influences the structure and function of the vascular network in the brain organoids.
Make a Comment
To make a comment you must login or register.