'Organ on a Chip' Models the Ins and Outs of the Blood-Brain Barrier
Quick Links
Infinitely more complex than a mere wall, the blood-brain barrier controls the passage of most solutes that enter or leave the brain. To get a handle on how this molecular sieve influences neural activity, researchers led by Donald Ingber and Kevin Kit Parker at Harvard University modeled the interactions between blood vessels and brain tissue on a series of interconnected “organ chips.” These are small polymer surfaces etched with hollow channels in which cells can grow and fluids can flow. When connected through this blood-brain-barrier (BBB) model, endothelial cells, pericytes, astrocytes, and neurons alter their gene expression, they authors show. They also report a new type of metabolic coupling between the BBB and neurons—metabolites released from the vascular endothelium influence the production of key neurotransmitters. The findings were published August 20 in Nature Biotechnology.
- Interconnected hollow channels mimic blood vessels and the brain parenchyma.
- The blood-brain-barrier model responds to drugs, such as methamphetamine.
- Vascular cells in the model modulate production of neurotransmitters by neurons.
“Parker’s multi-chip system has the potential to address several key topics of importance to AD, including Aβ clearance, the relationship between altered systemic metabolism and brain metabolism, the role of circulating factors in in neuronal inflammation and metabolism and vice versa, how neuronal factors might affect BBB physiology, and brain-derived biomarkers,” wrote Cheryl Wellington and Jérôme Robert of the University of British Columbia in Vancouver.
Joanna Wardlaw of the University of Edinburgh noted that while this technology might aid in studies of neurodegeneration, scientists have to be very cautious in their use and interpretation. “Cell behavior is influenced strongly by how cells interact with each other, so if something is missing the cells will behave differently,” she wrote.
Several in vitro models have been generated to study the BBB, including transwell cultures of endothelial and brain cells, artificial blood vessels, and, similar to this latest creation, microfluidic organ chips (Griep et al., 2013; Prabhakarpandian et al., 2013; Oct 2017 news; Brown et al., 2015). These models have started integrating an ever-expanding menagerie of cells that live both in and around the barrier, from the endothelial cells that line vessel walls to the pericytes, astrocytes, and neurons in the brain. For most, the vessel and brain components cannot be disconnected, making it difficult to disentangle the contributions of different cell types to overall neurovascular unit (NVU) function.
Co-first authors Ben Maoz, Anna Herland, and Edward FitzGerald addressed this problem by modeling the NVU on three separate chips that could be studied when connected or individually. The three chips modelled flow from blood vessels into the brain, flow through the cerebrospinal fluid in the brain, and flow from the brain back out into the vessels. The BBB influx chip was lined with human brain microvascular endothelial cells (hBMVECs) on the bottom and sides to represent a brain blood vessel, while an upper chamber, separated by a porous membrane, contained a mixture of pericytes and astrocytes to mimic the perivascular space just outside of the vessel. The brain chip was populated with a mix of neurons and astrocytes to mimic the parenchyma. Lastly, the efflux chip contained the same cells as the influx BBB chip. When connected, the three chips modeled the flow of solutes from the blood into the brain, and out again (see image below).
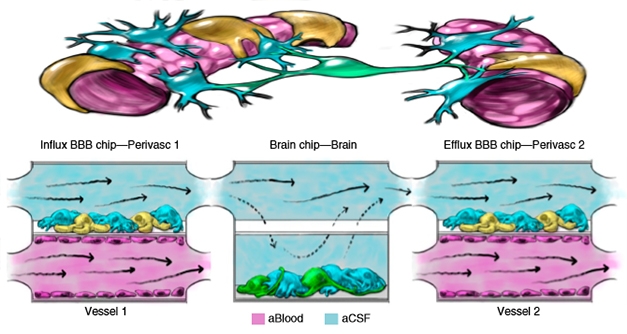
BBB Triple Play. In the brain (top left), solutes flow from endothelial cells (purple) and pericytes (yellow) of blood vessels through astrocytes (blue), to neurons (green), and back out again (right). Three linked microfluidic chips (bottom) model this flow. [Courtesy of Maoz et al., Nature Biotechnology, 2018.]
In keeping with BBB function, the researchers found that the small, brain-permeant dye Cascade blue could pass between the chips, while larger molecules, such as antibodies, remained trapped in the first blood vessel chip. The researchers also validated the NVU system by injecting methamphetamine into the BBB influx chamber. As has been shown before, the stimulant transiently broke down the barrier, allowing antibodies to pass from the influx chip into the brain chip.
With these indications of a working BBB model, the researchers next assessed how coupling of the different compartments would affect the expression of proteins in each one. Using mass spectrometry, the researchers observed significant changes to the proteomes of each compartment when they linked them together. For example, in the perivascular compartment, fluidic coupling boosted levels of proteins involved in amino acid and protein biosynthesis. In all compartments, coupling reduced levels of proteins involved in migration and proliferation, but boosted metabolism-associated proteins.
The researchers next used mass spectrometry to study how metabolites secreted by cells in each compartment affected the NVU. In a nutshell, the metabolome of the BBB as a whole strongly swayed the activity of neurons in the brain chip. For example, they found that glutamate, a synaptic transmitter usually associated with neurons, could also be produced in endothelial cells, then transported into the brain chip. On the other hand, GABA, another neurotransmitter, was exclusively produced within the brain chip. However, synthesis of GABA in the brain compartment was strongly influenced by levels of glutamate and other metabolites transported across the endothelium.
“The finding that the endothelium can produce metabolic products that serve as substrates for neurotransmitters produced by brain cells raises the interesting possibility that one might be able to modulate brain function by targeting the brain endothelium,” wrote Ingber in an email to Alzforum. “The advantage here is that the drug would not have to cross the blood-brain barrier, which could be a significant plus.”
How might this system facilitate the study of neurodegenerative disease? Ingber said the approach could be particularly useful to study how factors outside of the brain, including immune cells, drugs, and toxins, might influence neurodegenerative processes. He added that the incorporation of patient-derived induced pluripotent stem cells (iPSCs) into the devices could lend itself to personalized medicine.
Per-Ola Freskgard of Roche in Basel called the study an important step forward in modeling the BBB in vitro. “Hopefully it means that we can stop using the simplistic transwell systems that have been around for decades, especially when studying more complex aspects of the human neurovascular unit,” he said. He pointed out that the three linked chambers proved useful in sorting out contributions from each compartment. “However, a system that brings the different functional parts closer, or into a signal unit, would potentially be more advantageous to recapitulate the in vivo architecture, but still have the opportunity to study the different compartments separately,” he added.
Costantino Iadecola of Weill Cornell Medical College in New York pointed out that the cellular composition and functional characteristics of the NVU vary substantially throughout the cerebrovascular tree, and that Parker’s model is best suited to model capillaries. “Overall, this is a step forward in capillary NVU modeling, which, owing to IPS cell technology, affords the opportunity of more faithfully recapitulating selected aspect of human diseases,” he wrote (see full comment below).—Jessica Shugart
References
News Citations
Paper Citations
- Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, Couraud PO, Vermes I, van der Meer AD, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013 Feb;15(1):145-50. PubMed.
- Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013 Mar 21;13(6):1093-101. PubMed.
- Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015 Sep;9(5):054124. Epub 2015 Oct 26 PubMed.
Further Reading
Papers
- Gastfriend BD, Palecek SP, Shusta EV. Modeling the blood-brain barrier: Beyond the endothelial cells. Curr Opin Biomed Eng. 2018 Mar;5:6-12. PubMed.
- Bosworth AM, Faley SL, Bellan LM, Lippmann ES. Modeling Neurovascular Disorders and Therapeutic Outcomes with Human-Induced Pluripotent Stem Cells. Front Bioeng Biotechnol. 2017;5:87. Epub 2018 Jan 30 PubMed.
Primary Papers
- Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segrè D, Budnik B, Ingber DE, Parker KK. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol. 2018 Aug 20; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of British Columbia
University Hospital of Zurich
In this paper in Nature Biotechnology, Kit Parker and colleagues, led by Ben Maoz, Anna Herland, and Edward FitzGerald, describe an elegant platform using three linked organs-on-chips to model the human neurovascular unit (NVU) and the metabolic coupling of endothelial and neuronal cells.
The NVU is unquestionably a critical component of brain health, as it is the interface between central nervous system (CNS) and the rest of the body. The NVU has particular importance to Alzheimer’s disease as several cardiovascular disease risk factors also increase AD risk, and as many elements of AD pathophysiology impinge on the NVU, including Aβ clearance, blood-brain-barrier dysfunction, and neuroinflammation.…More
However, there are many challenges to study the NVU, as both in vivo and current in vitro approaches have several limitations. Parker’s group now provides an innovative approach to study the individual contributions of brain microvascular endothelial cells, perivascular pericytes, astrocytes, and neurons. His approach connects two blood-brain-barrier (BBB) chips to a brain chip, such that separation of the artificial blood and artificial cerebrospinal fluid (CSF) media is preserved. Essentially, when the chips are coupled, artificial blood flows through the endothelium lumen of the first BBB chip (BBB influx), and metabolic factors that are actively transported or perfuse through the perivascular compartment of the BBB influx chip are transferred to the brain chip, where it contacts a mixed culture of neurons and astrocytes. This fluid is then coupled to the perivascular compartment of the BBB efflux chip, where both artificial CSF and blood components can be sampled after contact with the brain compartment.
Several important studies were performed within this paper to validate the model. First, Maoz and colleagues used proteomic and metabolomic approaches to investigate protein expression in coupled versus uncoupled chips and demonstrated that fluidic coupling upregulated metabolism-associated proteins in all compartments and downregulated proteins associated with proliferation and migration compared to uncoupled chips. These data add considerably to a growing body of literature that cellular phenotypes depend on the microenvironment context, and that the traditional reductionist approaches of modeling individual cells is likely to be fraught with error.
Second, the utility of the linked chip platform for drug modeling studies was confirmed using methamphetamine, which is known to transiently disrupt the BBB after acute administration both in vivo and in vitro.
Finally, metabolomic analyses were performed to characterize the secretome produced by each compartment when coupled versus uncoupled. Here, BBB chips were found to be highly metabolically active and secrete chemical cues to maintain neuronal function, including regulating glutamate and gamma-aminobutyric (GABA) neurotransmitters. Analysis of the metabolic contribution of each compartment to the NVU system to pathways associated with glycolysis, the TCA cycle and the glutamine-glutamate cycle shows that glycolysis occurs in all compartments, glutamine synthesis was observed in all compartments containing astrocytes, and GABA is produced exclusively in the brain chip and is significantly higher in the coupled configuration. These data show that metabolites from the vascular endothelium and perivascular cells can directly affect glutamine production by astrocytes and neurons on the brain chip and influence the metabolism of neurons and astrocytes.
Parker’s multichip system has the potential to study several key topics of importance to AD, including Aβ clearance, the relationship between altered systemic metabolism and brain metabolism, the role of circulating factors in neuronal inflammation and metabolism and vice versa, how neuronal factors might affect BBB physiology, and brain-derived biomarkers.
The limitations of traditional in vitro systems to preserve the metabolic and functional state of cells is increasingly recognized, with several more advanced human multicellular models emerging (Brown et al., 2016; Robert et al., 2017). Although all of these systems have limitations, they represent valuable approaches to investigate human cellular physiology in the appropriate context and complement in vivo studies.
References:
Brown JA, Codreanu SG, Shi M, Sherrod SD, Markov DA, Neely MD, Britt CM, Hoilett OS, Reiserer RS, Samson PC, McCawley LJ, Webb DJ, Bowman AB, McLean JA, Wikswo JP. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J Neuroinflammation. 2016 Dec 12;13(1):306. PubMed.
Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A, Stukas S, Zhao W, Kulic I, Wellington CL. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. Elife. 2017 Oct 10;6 PubMed.
Weill College Medicine, New York
I am impressed by the fact that this model, unlike previous ones, provides the opportunity to study the interactions between different cellular compartments of the neurovascular unit (NVU) at the levels of capillaries. This system seems well-suited to examine transport and metabolic coupling among the different NVU cells at the capillary level, since smooth muscle cells and perivascular cells (macrophages, mast cells, etc.) are not included in the model.
The NVU is not uniform across the cerebrovascular tree. The cellular composition, signaling mechanism, and functional characteristics vary depending on the cerebrovascular segment examined (large arteries, pial arteries, penetrating arterioles, capillaries, venules and veins). It would be desirable if, in future iterations of the model, the basement membranes were also included. Much of the cerebrovascular pathobiology of cognitive impairment has been recently linked to basement membrane proteins and enzymes (CADASIL, CARASIL, small vessel disease, etc.). Overall, this is a step forward in capillary NVU modeling, which, owing to IPS cell technology, affords the opportunity to more faithfully recapitulate selected aspects of human diseases.…More
Make a Comment
To make a comment you must login or register.