Imaging Data Resurrects Abandoned Parkinson’s Gene Therapy
Quick Links
Researchers often do not understand exactly how treatments produce benefits. Take a recent gene therapy for Parkinson’s disease. It was intended to dampen excess activity in the subthalamic nucleus, mimicking the effect of deep brain stimulation (DBS). Indeed, in a small Phase 2 trial, the gene therapy modestly improved its recipients’ ability to move. But it did not work better than DBS and was not considered worth further development. Now, years later, researchers led by David Eidelberg at the Feinstein Institute for Medical Research, Manhasset, New York, report that the therapy acted in an unexpected fashion. Analyzing longitudinal FDG PET scans of brain glucose metabolism, they discovered a new brain network, which was absent in sham-treated placebo controls. The gene therapy appears to have activated this network. Its strength correlated with the amount of improvement in motor symptoms, and it could serve as an efficacy biomarker in future trials, Eidelberg said. The findings have sparked plans by a new company to take the therapy into a Phase 3 trial.
- A gene therapy for PD triggers formation of a new functional brain network.
- The network correlates with clinical improvement.
- The finding has revived industry interest in this therapeutic approach.
“We are really at the beginning of understanding how different treatments affect the brain,” Eidelberg told Alzforum. “Often, treatments achieve benefits in different ways than we expected.” He believes brain imaging tools now available will help optimize treatments for neurodegenerative disease.
This particular gene therapy approach was conceived by Matthew During at Ohio State University in Columbus and Michael Kaplitt at Weill Cornell Medical College, New York, who co-founded the biotech start-up Neurologix Inc. to develop it. (This is not to be confused with the Cleveland Clinic spin-off NeuroLogix Technologies, which develops therapies for traumatic brain injury.) To squelch noise in the subthalamic nucleus (STN), the scientists delivered the gene for glutamic acid decarboxylase (GAD) on an adenoviral vector. Because this enzyme produces the inhibitory neurotransmitter GABA, the approach should turn some excitatory neurons into inhibitory neurons and cool overall STN activity, the scientists reasoned. The approach appeared safe in Phase 1 (Kaplitt et al., 2007).

New Connections. Gene therapy leads to the formation of a new brain circuit (dark blue and red lines) in treated Parkinson’s patients (left), compared with sham-treated controls (right). [Courtesy of Science Translational Medicine/AAAS.]
In the Phase 2 trial, conducted between 2008 and 2010, 16 participants who received gene therapy enjoyed better motor control after six months than did 21 controls who underwent sham surgery (Mar 2011 news). The improvement persisted at a one-year open-label follow-up (Niethammer et al., 2017). The 25 percent improvement in UPDRS motor symptoms was not enough to entice investors, however. A planned Phase 3 trial fell through, and Neurologix Inc. went belly-up in 2012 (see story).
When Eidelberg took a closer look at the imaging data, however, he was in for a surprise. In treated people, he had expected to see a decrease in the abnormal metabolic network activity associated with Parkinson’s, dubbed the PD-related covariance pattern (PDRP). Both dopamine replacement therapy and deep brain stimulation suppress this aberrant network. Lo and behold, gene therapy did not. The PDRP pattern continued to worsen at the same rate in treated and untreated participants.
Instead, new brain regions began to talk to each other in treated participants. First author Martin Niethammer identified five new connections that had the net effect of better linking basal ganglia regions to the cortical premotor and motor regions. For example, the left superior frontal node connected to the left caudate nucleus, the right superior frontal node to the right supramarginal gyrus in the parietal lobe, and the left anterior putamen and globus pallidus to the ipsalateral thalamic node (see image above). Rather than using conventional motor pathways in the basal ganglia and thalamus, these new connections co-opted nearby nonmotor regions in each structure, Niethammer realized. In addition, metabolic activity surged in the premotor region and supramarginal gyrus compared with controls, while cooling in the basal ganglia, the ventral anterior and medial dorsal thalamic nuclei, and the inferior frontal gyrus.
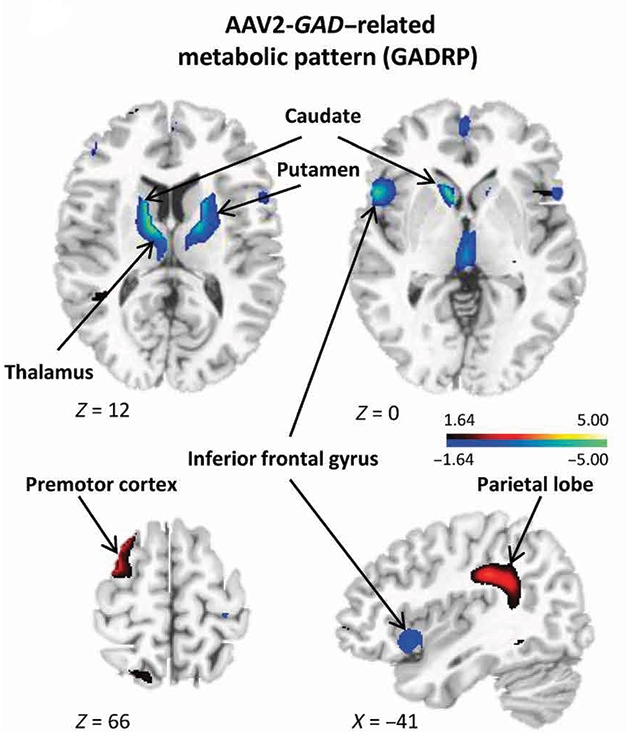
Activity Changes. In gene therapy recipients, metabolic activity waned in some regions (blue) and heated up in others (red) compared with sham-treated controls. [Courtesy of Science Translational Medicine/AAAS.]
Eidelberg noted that in Parkinson’s, brain activity in the basal ganglia becomes chaotic due to the loss of dopamine signaling. “Gene therapy allows the brain to bypass this noisy, overactive mess and reach the motor cortex through other means,” he said. He thinks increased inhibitory signaling from the STN pushes other synapses to reorganize their activity, forming fresh connections.
This new network, called the GAD-related covariance pattern (GADRP) appeared at six months, and heightened by one year after surgery. Eidelberg believes the network continues to mature as people use their motor skills. As the network gradually becomes more efficient, it may stabilize the clinical benefit, he suspects. The GADRP was specific to the gene therapy. It was present in all treated participants, but did not appear in people who took dopamine medication or received DBS.
The researchers were able to distinguish GADRP from the placebo effect, which is strong in Parkinson’s trials because the expectation of a good outcome itself activates the dopamine system. In both the treatment and sham groups, researchers saw activation of a different novel network that mostly involved the limbic system and cerebellum. The strength of this network correlated with a modest motor benefit seen in the placebo group, but bore no relationship to clinical improvement in the treatment group. Eidelberg speculates that the much stronger effect of GADRP swamped out the effect of the placebo network in people who received the GAD gene.
Based on these new imaging data, a British gene therapy company has acquired the rights to the AAV2-GAD therapy, Eidelberg said. He declined to name it because the company has not yet made a public announcement.
Hideki Mochizuki at Osaka University, Japan, agrees the therapy is worth pursuing, but urged caution. “The findings are interesting, and personally I still think that GAD delivery is a promising gene therapy. But its clinical outcome should be compared with deep brain stimulation in terms of safety and efficacy before wide-range application could be envisaged,” he wrote to Alzforum.
Eisenberg believes that the GADRP's correlation with motor improvement makes it attractive as a quantitative readout that would be more consistent and less noisy than clinical ratings. “We’ll be able to see quickly if this intervention helps people,” Eidelberg said. He believes a similar approach could potentially work with other types of PD therapy, although each intervention might activate distinct brain network changes.
Researchers have explored gene therapy approaches for PD in the clinic on and off since 2007 (for review see Axelsen and Woldbye, 2018). Some try to bolster flagging dopaminergic neurons with growth factors. The only one that has completed a human study, neurturin, showed no consistent benefit in randomized trials (Marks et al., 2010; Warren Olanow et al., 2015). A Phase 1 study of the related growth factor GDNF is underway. Others attempt to replace dopamine by infusing the three genes responsible for its production. A Phase 1 extension study of this method is ongoing, with the treatment well-tolerated so far (Jan 2014 news).
Yet other groups infuse the enzyme aromatic acid decarboxylase (AADC), which converts levodopa to dopamine, into the putamen. This approach helps patients better utilize levodopa medication, reducing dyskinesia and lengthening periods of good motor control (Mar 2018 news). Earlier this month, Voyager Therapeutics announced that these benefits are maintained long-term (see company press release), and plans to take the therapy to a Phase 2 trial.—Madolyn Bowman Rogers
References
News Citations
- First Phase 2 Success for Gene Therapy in Parkinson’s
- Parkinson's: Update on Gene Therapy, Fetal Cell Transplants
- So Far, So Good for Parkinson’s Gene Therapy
Paper Citations
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007 Jun 23;369(9579):2097-105. PubMed.
- Niethammer M, Tang CC, LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Sapan CV, Eidelberg D, During MJ, Kaplitt MG, Feigin A. Long-term follow-up of a randomized AAV2- GAD gene therapy trial for Parkinson's disease. JCI Insight. 2017 Apr 6;2(7):e90133. PubMed.
- Axelsen TM, Woldbye DP. Gene Therapy for Parkinson's Disease, An Update. J Parkinsons Dis. 2018;8(2):195-215. PubMed.
- Marks WJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010 Dec;9(12):1164-72. PubMed.
- Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, Kordower JH, Alterman R, Lozano AM, Lang AE. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol. 2015 Aug;78(2):248-57. Epub 2015 Jun 10 PubMed.
External Citations
Further Reading
Primary Papers
- Niethammer M, Tang CC, Vo A, Nguyen N, Spetsieris P, Dhawan V, Ma Y, Small M, Feigin A, During MJ, Kaplitt MG, Eidelberg D. Gene therapy reduces Parkinson's disease symptoms by reorganizing functional brain connectivity. Sci Transl Med. 2018 Nov 28;10(469) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.