Gene Therapy Treats Mouse Models of Gaucher’s Disease
Quick Links
Babies with an incurable, fatal form of Gaucher’s disease are born with severe irreversible neurodegeneration and typically die before age 2. According to a new paper in the July 16 Nature Medicine, replacing a defunct gene at the fetal stage may prevent this devastating disorder. Researchers led by Simon Waddington and Ahad Rahim of University College London used a viral vector to introduce a working copy of glucocerebrosidase into mouse fetuses that lack the enzyme. The researchers saw vastly reduced neuroinflammation and neurodegeneration in newborn pups, accompanied by a major extension of lifespan.
- Fetal gene therapy rescues mouse models of Gaucher’s disease.
- Treatment reduces neuroinflammation, neurodegeneration, and damage to visceral organs.
- Treatment also works in newborns.
“The paper was really encouraging,” said Penelope Hallett, Harvard Medical School, who was not involved in the research. “It’s nice to see that this therapy has such a dramatic effect.” Given the current lack of treatment for neuronopathic Gaucher’s disease (nGD), this has potential for translation to the clinic, she said. In addition, it means that other lysosomal storage disorders that affect children may be amenable to this kind of gene therapy, she told Alzforum.
Gaucher’s disease is an autosomal recessive disorder caused by two faulty copies of the GBA gene that encodes glucocerebrosidase (GCase). This lysosomal enzyme breaks down glucosylceramide, a normal component of the cell membrane. Heterozygous mutations in the gene for GCase raise the risk for Parkinson’s disease, because they exacerbate misfolding and accumulation of α-synuclein aggregates, which in turn gum up GCase activity in the lysosome (Jun 2011 news). In GD, glucosylceramide builds up in the liver, spleen, bone marrow, and nervous system. The disease takes several forms: a milder version, known as Type 1, primarily hits the organs outside the brain and can be treated by regular intravenous injections of GCase; Type 2 also affects the hindbrain, causing neurodegeneration. Because GCase cannot cross the blood-brain barrier, the neuronopathic form is more serious. Could gene therapy restore enzyme function and cure the disease?
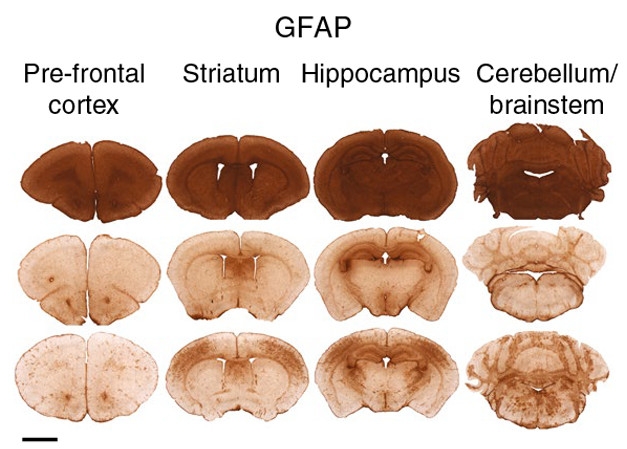
Fetal Gene Therapy Rescues nGD Mice. Astrogliosis (brown) plagues the brains of 12-day-old GCase knockouts (top) compared with 35-day-old wild-type mice (middle). Fetal GCase gene delivery (bottom) suppresses astrogliosis in 35-day-old knockouts (bottom). [Image courtesy of Massaro et al., 2018. Nature Med.]
To find out, first author Giulia Massaro and colleagues turned to a mouse model of nGD that has both copies of the GCase gene knocked out. At birth, these mice already have extensive microglial activation and astrogliosis in the brainstem, which both extend to the entire brain by day 12. Widespread neurodegeneration affects the cortex, thalamus, and brainstem by then, also. Glucosylceramides increase up to 22-fold in the brain. Untreated, these mice die in just two weeks.
Massaro operated on pregnant female mice, injecting their fetuses intracranially with an AAV9 virus that contained the human GCase gene. The treated pups lived much longer than untreated controls, surviving the entire 19 weeks of the study, most still appearing healthy and some even fertile. At 30 days of age, GCase appeared throughout the brain.
The treated mice weren’t completely cured, however. While they had substantially less microglial activation, astrogliosis, and neurodegeneration than controls, those measures were still slightly elevated. The mice fell from a rotating cylinder more easily than did wild-type mice and lost their footing while walking on a mesh grid. They ran around more and weighed less. Though glucosylceramide hadn’t built up in the brain, shorter chain glycosphingolipids had.
Nevertheless, the treatment had a substantial impact, said Waddington. “The efficacy we could achieve with early gene therapy was stunning,” he told Alzforum. “This is probably the most emphatic therapeutic efficacy we’ve seen in any mouse model of a disease using fetal gene therapy.”
Treating human babies in the womb would be ideal, given that neurodegeneration is already extensive by birth in Type 2 disease, but it is technically challenging, said Waddington. Treating newborns might be more attractive for companies looking to develop this kind of therapy. To test whether this gene therapy would work in newborn mice, the authors injected day-old GCase knockout pups either intracranially or in a vein in the skull with the same AAV9-GCase vector.
In both cases, pups survived for 55 days, the length of the experiment, at which point GCase was active throughout the brain. However, while neuroinflammation and neuron loss were substantially reduced at day 55, they still cropped up in some regions. This could be because of variability in the extent of transduction of neurons with the vector, said Hallett. Injected into a vein, the AAV vector also reduced cell damage to the spleen, liver, and lungs.
Despite being less effective than gene therapy in utero, treating newborns may be adequate, said Ellen Sidransky, National Human Genome Research Institute, Bethesda, Maryland. “Mice had an improved life expectancy, enzyme activity in the brain increased, and intravenous delivery corrected visceral symptoms and motor function. If these positive effects are even partially recapitulated in humans, this would make a big difference for babies affected by Type 2 Gaucher disease, and even potentially for patients with milder disease,” she told Alzforum.
Hallett said that the work supports the idea that normalizing lysosomal hydrolases such as GCase could be relevant for Parkinson’s disease (PD) or dementia with Lewy bodies, since as many as half of PD patients carry a mutation in a lysosomal storage disorder gene (Robak et al., 2017). “It’s all supportive of using gene therapy to increase lysosomal enzymes for preventing or reducing neurodegeneration,” she said. Gene therapies have been tested for other targets in PD, including boosting levels of nerve growth factor and more recently dopamine (Mar 2018 news). A small molecule approach for suppressing glucosylceramide synthesis is in Phase 2 for PD patients with GBA mutations (Dec 2016 news).
Waddington is now working with Apollo Therapeutics in Stevenage, U.K., to optimize an intravenous gene delivery system that will treat both the neurological and visceral symptoms of nGD in babies. At first, they will likely treat newborns, given the difficulties of treating fetuses, he told Alzforum. The ultimate goal is to deliver the gene to the fetus, he said. Doing so should have the added bonus of avoiding immune responses to the AAV9 vector, he added, since the immature immune system of a fetus is more likely to accept an AAV as “self.”—Gwyneth Dickey Zakaib
References
News Citations
- Feedback Loop—Molecular Mechanism for PD, Gaucher’s Connection
- So Far, So Good for Parkinson’s Gene Therapy
- Large Phase 2 Trial Starting Up in Genetic Parkinson’s Population
Paper Citations
- Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, International Parkinson’s Disease Genomics Consortium (IPDGC), Heutink P, Shulman JM. Excessive burden of lysosomal storage disorder gene variants in Parkinson's disease. Brain. 2017 Dec 1;140(12):3191-3203. PubMed.
Further Reading
Papers
- Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, Kissel JT, Nagendran S, L'Italien J, Sproule DM, Wells C, Cardenas JA, Heitzer MD, Kaspar A, Corcoran S, Braun L, Likhite S, Miranda C, Meyer K, Foust KD, Burghes AH, Kaspar BK. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. 2017 Nov 2;377(18):1713-1722. PubMed.
- Mulcahy PJ, Iremonger K, Karyka E, Herranz-Martín S, Shum KT, Tam JK, Azzouz M. Gene therapy: a promising approach to treating spinal muscular atrophy. Hum Gene Ther. 2014 Jul;25(7):575-86. Epub 2014 Jun 27 PubMed.
Primary Papers
- Massaro G, Mattar CN, Wong AM, Sirka E, Buckley SM, Herbert BR, Karlsson S, Perocheau DP, Burke D, Heales S, Richard-Londt A, Brandner S, Huebecker M, Priestman DA, Platt FM, Mills K, Biswas A, Cooper JD, Chan JK, Cheng SH, Waddington SN, Rahim AA. Fetal gene therapy for neurodegenerative disease of infants. Nat Med. 2018 Sep;24(9):1317-1323. Epub 2018 Jul 16 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.