To Deliver Itself From Cell to Cell, Phospho-Tau Uses UPS
Quick Links
When idle, tau may have nothing better to do than hitch a ride out of town. According to a study in the May 15 Cell Reports, phosphorylated tau, which can no longer bind microtubules, instead interacts with the inner leaflet of the plasma membrane. Tau then translocates across the membrane and, with the help of sulfated proteoglycans on the other side, escapes into the extracellular space. The authors, led by Walter Nickel and Thomas Jahn at the University of Heidelberg in Germany, reported that once released by this unconventional secretion mechanism, tau managed to enter neighboring cells and trigger aggregation. The experiments were restricted to cultured cells overexpressing tau. Even so, they offer a potential mechanistic link between tau hyperphosphorylation and its subsequent propagation in the brain.
- Tau phosphorylation triggers detachment from microtubules and release from cells.
- Tau escapes by a type I unconventional protein secretion pathway.
- Secreted tau is taken up by neighboring cells and stokes aggregation there.
Tau’s primary role as a microtubule stabilizer places the protein inside the cell, and the tau tangles that crop up in Alzheimer’s disease also accumulate intracellularly. However, tau clearly has extracellular dalliances. The protein rises in cerebrospinal fluid in the preclinical stages of AD, and somehow propagates between neurons in the brain (Apr 2015 conference news; Mar 2018 news). Previous studies have reported that neuronal excitation triggers tau’s release (Aug 2013 news; Feb 2014 news; Jun 2016 news). Tau lacks the signal sequence required for secretion via the canonical ER-to-Golgi pathway, and several alternative routes have been proposed in recent years, including chaperone-assisted secretion and release via exosomes (Jun 2016 news; Oct 2015 news).
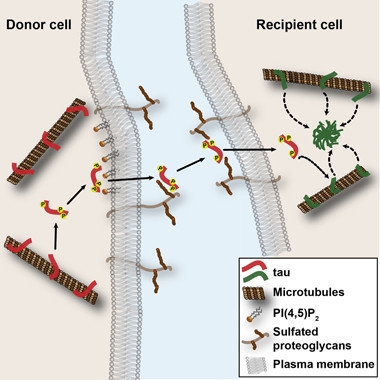
Tau Jumps Ship.
Phosphorylated tau dissociates from microtubules, associates with the plasma membrane, and grabs sulfated proteoglycans on the cell surface before transferring to neighboring cells, where it triggers aggregation. [Courtesy of Katsinelos et al., Cell Reports, 2018.]
First author Taxiarchis Katsinelos and colleagues set out to investigate. Because phosphorylation occurs early in tau’s pathogenic transformation, the researchers first asked if this modification affected its release. It did. They found that SH-SY5Y neuroblastoma cells only secreted tau-E14, a phosphomimetic. But how?
Only about 7 percent of the secreted tau associated with lipid vesicles, arguing against an exosomal secretion mechanism. The researchers hypothesized that tau secretion occurs via the type I unconventional protein secretion (UPS) pathway. Proteins using this route, including fibroblast growth factor 2 (FGF2) and HIV’s Tat peptide, associate directly with the plasma membrane, and with heparin sulfate proteoglycans (HSPGs) on the cell surface, to orchestrate their release. To see if tau did, too, the researchers treated the neuroblastoma cells with sodium chlorate, a compound that inhibits the final sulfation step needed for proteoglycan maturation. The treatment effectively blocked the release of tau-E14, implicating type I UPS in tau’s escape.
To more carefully dissect the molecular mechanisms involved, the researchers next used Chinese hamster ovary (CHO) cells, in which they overexpressed human tau. Compared with wild-type tau or a phosphorylation-deficient form, tau-AP, tau-E14 associated less strongly with microtubules purified from the cells. Tau-E14 formed insoluble aggregates within the cells, a phenomenon that might be due to its detachment from microtubules. In addition to the release of tau-E14 from the CHO cells, wild-type tau was secreted from CHO cells that overexpressed GSK3β, a kinase that phosphorylates tau. Most of the secreted tau was free from vesicles, soluble, and full-length.
In keeping with tau’s secretion via type I UPS, tau interacted with phospholipids similar to those that line the inner leaflet of the plasma membrane, though only when phosphoinositol (4,5) bisphosphate (PI(4,5)P2) was present. In CHO cells, treatment with the PI(4,5)P2 protein interaction inhibitor neomycin stymied tau release.
Besides seeing tau floating in the culture medium, the researchers also detected it clinging to the extracellular side of the membrane prior to release. They saw more of it when tau was phosphorylated. They hypothesized that surface HSPGs could be anchoring tau there. Previous studies had reported that these sticky cell-surface proteoglycans facilitate tau’s uptake and propagation, and even snag Aβ (Holmes et al., 2013; Apr 2016 news). Sure enough, the scientists found that either blocking sulfation with sodium chlorate or using CHO cells deficient in the HSPG sulfated glycosaminoglycan (sGAG) knocked down levels of cell-surface tau by a third. In the media, sGAG deficiency reduced levels of free tau even more, suggesting that the HSPGs serve as an anchor point on the extracellular surface prior to tau’s leap into the extracellular abyss. Notably, tau-E14 release returned to near normal levels when the researchers added sGAG-replete CHO cells into the culture, suggesting that HSPGs on neighboring cells could aid in the release of tau from cells lacking the proteoglycans on their own surface.
Could tau’s voyage via UPS facilitate its transfer into other cells? To find out, the researchers used co-culture. They mixed tau-E14-expressing CHO donor cells with CHO acceptor cells expressing green fluorescent protein (GFP) but no tau. With immunofluorescence microscopy, they saw tau-E14 transfer from the donor cells to 10 percent of the acceptor cells. The transfer process depended upon HSPGs.

Tau Transfer. Mixing cells expressing GFP (green) and HA-tagged Tau-E14 (red) shows that tau can transfer among cells (red dot, arrow, right). [Courtesy of Katsinelos et al., Cell Reports, 2018.]
Finally, the researchers asked whether tau in acceptor cells could stoke aggregation there. They co-cultured their tau-E14-expressing CHO cells with cells from a biosensor line similar to the one developed by Marc Diamond at University of Texas Southwestern Medical Center in Dallas (May 2014 news). The biosensors in this study express a fluorescently tagged tau-repeat domain, which forms distinct clusters upon aggregation. Tau-E14-expressing cells indeed triggered tau aggregation in the sensor cells, suggesting the secreted tau acted as a proteopathic seed. Neither transfer nor aggregation happened when the donor cells lacked sGAG.
Jahn, now at Abbvie, told Alzforum that for other proteins the UPS secretion pathway serves a physiological role. However, for tau, it could be a biophysical accident, he explained, brought about by hyperphosphorylation and being cut loose from microtubules. Future experiments can address how the process unfolds in neurons in the brain, where neuronal activity and tau intraneuronal localization come into play.
Work led by Diamond also implicates HSPG in tau spread, at least in its uptake. He reported that the internalization of tau aggregates depended on both the length and sulfation of sGAGs on acceptor cells. Aβ and α-synuclein aggregates gained access to cells via sGAGs also, but were less discerning (e.g., March 2013 conference news; Stopschinski et al., 2018).
Khalid Iqbal of the New York State Institute for Basic Research in Staten Island commented that the secretion mechanism could help explain the spread of tau pathology in AD and other tauopathies. He said this mechanism suggests tau can spread by proximity, rather than just trans-synaptically.
On a similar note, Rakez Kayed of the University of Texas in Galveston noted that this elegant mechanistic study could explain local spread of toxic tau oligomers. He speculated that interaction with HSPGs could facilitate tau oligomerization on the plasma membrane, transforming it into a toxic form before being released to neighboring cells. Kayed added that while this secretion mechanism is likely important, others are sure to exist in the complex environment of the brain.—Jessica Shugart
References
News Citations
- Protein Propagation Real, but Mechanisms Hazy
- Isotope Labeling Links Tau Production to Aβ Burden
- Tales of Traveling Tau: Is Transfer Between Neurons Normal?
- Neurons Release Tau in Response to Excitation
- Excited Neurons Release More Aberrant Tau
- Ushers of Propagation? More Evidence that Chaperones Evict Disease-Associated Proteins
- Deadly Delivery: Microglia May Traffic Tau Via Exosomes
- Sticky Matrix Proteins Lead to Amyloid Accumulation, Slow Clearance
- Like Prions, Tau Strains Are True to Form
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
Paper Citations
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):E3138-47. Epub 2013 Jul 29 PubMed.
- Stopschinski BE, Holmes BB, Miller GM, Manon VA, Vaquer-Alicea J, Prueitt WL, Hsieh-Wilson LC, Diamond MI. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. J Biol Chem. 2018 Jul 6;293(27):10826-10840. Epub 2018 May 11 PubMed.
Further Reading
Primary Papers
- Katsinelos T, Zeitler M, Dimou E, Karakatsani A, Müller HM, Nachman E, Steringer JP, Ruiz de Almodovar C, Nickel W, Jahn TR. Unconventional Secretion Mediates the Trans-cellular Spreading of Tau. Cell Rep. 2018 May 15;23(7):2039-2055. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.