Cerebrospinal Fluid from Youngsters Boosts Memory in Old Mice
Quick Links
The cerebrospinal fluid from young mice is awash with factors that keep the brain sharp. Now, scientists led by Tony Wyss-Coray at Stanford University in Palo Alto, California, report that when injected into the brains of old mice, CSF from young mice, or young people, revved expression of a host of oligodendrocyte genes within the hippocampus, counteracting the slump in proliferation and function of the myelin-making cells that typically occurs with age. As reported May 11 in Nature, the treatment also improved memory. The researchers tied the effects to fibroblast growth factor 17 in the young CSF, which increased expression of serum response factor in oligodendrocytes, enhancing their proliferation and myelin production.
- Cerebrospinal fluid from the young improves memory in older mice.
- It boosts oligodendrocyte numbers and myelin production in the hippocampus.
- Fibroblast growth factor 17 is partly responsible.
“This work demonstrates the rejuvenating capacity of young CSF, and highlights oligodendrocyte lineage cells as a potential target for therapeutic strategies to prevent age-associated cognitive decline,” wrote Hansruedi Mathys of the University of Pittsburgh.
The findings are the latest in a long line of studies from Wyss-Coray’s lab that identified factors in young animals that influence aging of the brain (for review, Pluvinage and Wyss-Coray, 2020). Previously, the group reported that human umbilical cord blood, and plasma from people in their 20s or from 3-month-old mice, boosts neurogenesis, neuronal plasticity in the hippocampus, and memory in aged mice (May 2014 news; Apr 2017 news; Sep 2019 news).
Compared to plasma, cerebrospinal fluid is far more intimately connected to brain. Churned out in the choroid plexus and imbued with neuroprotective factors, CSF works its way through the brain via the glymphatic system (Aug 2012 news). Its composition also changes with age. Might CSF components from the young rejuvenate the aging brain? First author Tal Iram and colleagues collected CSF and infused it directly into the right lateral ventricles of 20-month-old animals daily for a week. They started the infusions just after they gave the animals a foot shock and tested their memories three weeks after the shock. Compared to old mice infused with artificial CSF, those infused with the fluid from 3-month-old mice were more adept at remembering where they had previously received series of unpleasant foot shocks three weeks prior—a stretch of time considered “remote” for a mouse. This suggested that the young CSF improved the animals’ remote recall, a type of memory that depends on the hippocampus. Given the timing of the infusion, the researchers believe the CSF helped the mice consolidate memories.

Youthful Boost. In the hippocampi of aged mice, CSF from young people (top) evoked more proliferation of OPCs (green) as seen by incorporation of the thymidine analog EdU (red and arrowheads) than did CSF from older people (bottom). [Courtesy of Iram et al., Nature, 2022.]
To investigate this improvement, the researchers looked for changes in the hippocampal transcriptome using RNA-Seq. They found a rise in transcripts encoding oligodendrocyte genes, including those that drive differentiation of the myelin-producing cells from oligodendrocyte precursors (OPCs), as well as an uptick in expression of major components of myelin itself. Neither artificial CSF, nor CSF from aged mice, triggered these responses. Furthermore, the percentage of actively proliferating OPCs in the hippocampus more than doubled when mice were infused with young CSF. Notably, human CSF pooled from donors in their 20s triggered a similar OPC proliferative response, while CSF pooled from donors in their 70s only coaxed half as many cells to divide. Curiously, young CSF did not spur OPC proliferation in the cortex, suggesting that hippocampal OPCs were particularly responsive.
Did these proliferating OPCs mature into bona fide myelin-makers? Indeed, the researchers found that three weeks after infusion with young CSF, levels of myelin basic protein, as well as numbers of myelinated axons, had increased in the molecular layer of the hippocampus.
How did young CSF promote an oligodendrocyte renaissance in aged mice? A clue came from the first batch of transcripts to rise in response to CSF. Using metabolic labeling to tag freshly made transcripts in cultured OPCs, Iram found that within one hour of treatment with young CSF, expression of serum response factor (SRF) was more strongly upregulated than any other gene. Expressed in multiple organs throughout the body, SRF binds to serum response element (SRE) promoter sequences, activating genes that help coordinate cytoskeletal arrangements involved in all manner of cellular functions, including motility and proliferation. In neurons, SRF orchestrates the growth of axons and aids in synapse formation (Knöll et al., 2006). What role does the transcription factor play in oligodendrocytes? Iram said they are looking into that, but for the purposes of this study, the researchers found that SRF was necessary for OPCs to proliferate in response to young CSF. Moreover, expression of SRF in mouse hippocampal OPCs waned markedly as the animals aged. Digging through data from published human studies, the authors found that SRF expression also tapers in the brains of people with AD (Mathys et al., 2019; Zhou et al., 2020).
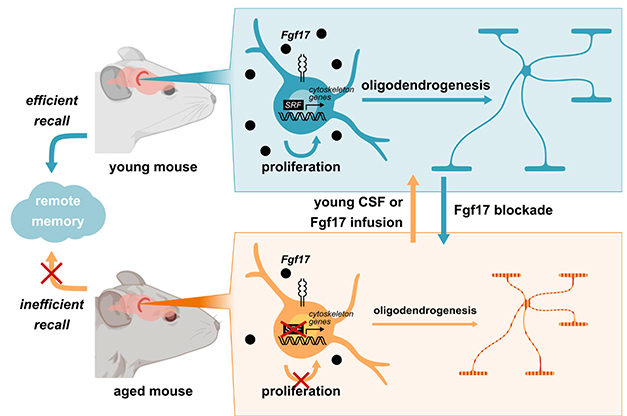
Young vs. Old. Abundant Fgf17 (black dots) in the CSF of a young mouse (top), promotes SRF-driven gene expression, enhancing oligodendrogenesis, myelination of axons, and sharp memory. In old mice, Fgf17 wanes, gene expression and myelin production fall, and memory falters (bottom). Infusions of young CSF or Fgf17 rejuvenates old mice (orange arrow), while blocking Fgf17 in young mice makes them behave like old (blue arrow). [Courtesy of Iram et al., Nature, 2022.]
What factors within young CSF dialed up SRF expression? The authors speculated that hundreds of proteins within CSF could do the trick. In fact, many SRF target genes also feedback to induce SRF itself, and the researchers leveraged this to whittle down the list of potential suspects. By cross-referencing CSF proteomics datasets with lists of known SRF target genes, they came up with 35 potentials. Among them, Iram found that two—Fgf8 and Fgf17—most strongly induced SRF expression in human kidney cells. Because it is preferentially expressed in the brain and reportedly drops in human CSF with age, the researchers investigated Fgf17 further. They found it expressed in neurons in the mouse cortex and hippocampus, and that this expression drops dramatically with age. However, they could not detect the growth factor in their mouse CSF samples. Still, when infused into aged mice, Fgf17 enhanced SRF expression in hippocampal OPCs, ramped up OPC proliferation, and even improved memory, just like the young CSF. Infusion of Fgf17 antibodies into young mice worsened the animals' performance on memory tests, suggesting the factor plays a broad role in memory formation. Notably, blocking the growth factor in OPC cultures stymied their proliferation.
In all, the findings suggest that Fgf17 and potentially other growth factors within young CSF promote critical functions of oligodendrocytes, and that loss of these factors with age may lead to the erosion of oligodendrocyte function and myelination. While myelin in the brain is mostly wrapped around axons early in life, recent studies suggest that fresh production of this fatty sheath, spurred by neuronal activity, is important for learning and memory throughout life (Feb 2020 news). Iram noted that myelin production increases into adulthood before starting to wane as people enter their 60s. Together, these studies implicate flagging oligodendrocyte function in age-related cognitive decline, she said.
To Ragnhildur Thura Káradóttir of the University of Cambridge, U.K., the findings add to mounting evidence that oligodendrocytes and myelin maintenance play a prominent role in age-related memory loss and neurological disease. In the past, the role of oligodendrocytes in these processes has been underappreciated, she noted. Iram’s study goes a step further, by showing that the brain’s environment—in this case, factors in the CSF—may be capable of driving, or slowing, these age-related changes, she told Alzforum. Káradóttir was intrigued that Fgf17 enhanced oligodendrocyte function, but thinks it likely only one piece of a much larger picture of CSF/oligodendrocyte relations.
Vivek Swarup of the University of California, Irvine, agreed that future work will be needed to paint a more comprehensive picture of how factors in CSF influence oligodendrocyte development and function. He wondered how the findings translate to what happens in Alzheimer’s and other neurodegenerative diseases. “Will young CSF also curb memory loss caused by neurodegenerative disease?” he asked. The answer to that question could be exciting from a therapeutic standpoint, he said.
Li-Huei Tsai and Leyla Akay of Massachusetts Institute of Technology agreed (comment below). “Understanding how SRF and Fgf17 promote myelination, and determining whether this can be leveraged to treat neurodegenerative diseases associated with demyelination and white-matter injury, will make for exciting future research,” they wrote.
In a Nature News & Views, Miriam Zawadzki and Maria Lehtinen of Boston Children’s Hospital noted that the paper raises a provocative hypothesis about protein and fluid distribution throughout the brain. “Unexpectedly, Iram and colleagues found that FGF17 in the CSF isn’t sourced by the choroid plexus, but, instead, by youthful neurons themselves, providing evidence that neuron-based signals are delivered by the CSF,” they wrote. “How FGF17 is distributed in the CSF and delivered to target cells in the hippocampus presents a new direction of research.”—Jessica Shugart
References
News Citations
- In Revival of Parabiosis, Young Blood Rejuvenates Aging Microglia, Cognition
- Umbilical Cord Blood Tied to Hippocampal Rejuvenation
- 'Runner Plasma' Jogs Neurogenesis, Quells Neuroinflammation in Mice
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- New Myelin Makes Memories, but Supply Wanes with Age
Paper Citations
- Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020 Feb;21(2):93-102. Epub 2020 Jan 8 PubMed.
- Knöll B, Kretz O, Fiedler C, Alberti S, Schütz G, Frotscher M, Nordheim A. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat Neurosci. 2006 Feb;9(2):195-204. PubMed.
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019 Jun;570(7761):332-337. Epub 2019 May 1 PubMed.
- Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, Poliani PL, Cominelli M, Grover S, Gilfillan S, Cella M, Ulland TK, Zaitsev K, Miyashita A, Ikeuchi T, Sainouchi M, Kakita A, Bennett DA, Schneider JA, Nichols MR, Beausoleil SA, Ulrich JD, Holtzman DM, Artyomov MN, Colonna M. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat Med. 2020 Jan;26(1):131-142. Epub 2020 Jan 13 PubMed. Correction.
Further Reading
Primary Papers
- Iram T, Kern F, Kaur A, Myneni S, Morningstar AR, Shin H, Garcia MA, Yerra L, Palovics R, Yang AC, Hahn O, Lu N, Shuken SR, Haney MS, Lehallier B, Iyer M, Luo J, Zetterberg H, Keller A, Zuchero JB, Wyss-Coray T. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature. 2022 May;605(7910):509-515. Epub 2022 May 11 PubMed. Correction.
Annotate
To make an annotation you must Login or Register.

Comments
University of Pittsburgh
I very much enjoyed reading this outstanding paper from the Wyss-Coray lab. The discovery that infusing young CSF into aged brains improves memory function and boosts OPC proliferation and differentiation is fascinating and a very important contribution. This work demonstrates the rejuvenating capacity of young CSF and highlights oligodendrocyte lineage cells as a potential target for therapeutic strategies to prevent age-associated cognitive decline.
MIT
Picower Institute of MIT
In this exciting paper from Tony Wyss-Coray's group at Stanford, researchers lead by Dr. Tal Iram found that cerebrospinal fluid isolated from young mice stimulates oligodendrocyte precursor proliferation and differentiation, and promotes myelination in aged mice. Infusion of "young" CSF into aged mice also improved learning in a contextual memory paradigm. Interestingly, the transcription factor Serum Response Factor (SRF), and the growth factor Fgf17, were identified as putative mediators of these effects.
This important study adds to a growing body of literature highlighting oligodendrocyte lineage cells as active contributors to brain function, and potential drivers of disease. Understanding how SRF and Fgf17 promote myelination, and determining whether this can be leveraged to treat neurodegenerative diseases associated with demyelination and white-matter injury, will make for exciting future research.…More
Third Military Medical University
Oligodendrogenesis: A Brake on Brain Aging?
Interventions to slow down cognitive decline are sorely needed for the increasing elderly population worldwide. This recent Nature paper reported that perfusing aged mouse brains with young cerebrospinal fluid (CSF) is effective to improve memory capacity. Noticeably, a surge of oligodendroglial proliferation and differentiation (oligodendrogenesis) is the most prominent phenomenon in the aged mouse hippocampus after treatment with the young CSF. Oligodendrogenesis has been shown to be required for multiple brain functions, such as learning new skills and remote memory consolidation, which is greatly diminished in aged brains.
In addition, the authors identified fibroblast growth factor 17 as the key player in boosting oligodendrogenesis in vivo and in vitro. Manipulations of this signal pathway were sufficient to duplicate the effects of CSF on oligodendrogenesis and memory capacity. In line with this notion, previous studies have shown that enhancing myelination, either by drugs or by cell-specific manipulation, is enough to boost the performance of aged mice in memory-related tasks.…More
Together, these results indicate that oligodendrogenesis plays a pivotal role in slowing down age-related functional decline, underlining flourishing oligodendrogenesis as a promising way to put a brake on brain aging.
TrueBinding
This is an exciting paper by Tony Wyss-Coray's group, investigating the role of the transcription factor Serum Response Factor (SRF), and the growth factor Fgf17, in the CSF. Their ability to restore oligodendrocytes and improve cognition is fascinating. If I remember correctly, Wyss-Coray published a paper in 2014 in which he demonstrated that exposure to young blood counteracts aging at the molecular, structural, functional, and cognitive levels in the aged hippocampus. They identified CREB in young blood as one member of the regulatory network underlying structural and cognitive enhancements.…More
It will be interesting to investigate the mechanism of action SRF and Fgf17 and the role of these proteins in neurodegenerative diseases associated with traumatic brain injury or spinal cord injury.
View all comments by Suhail RasoolMake a Comment
To make a comment you must login or register.