Blood-Brain Barrier Surprise: Proteins Flood into Young Brain
Quick Links
The blood-brain barrier keeps most plasma proteins out of the brain, but becomes leaky with age and in Alzheimer’s. Or so scientists thought. In the July 1 Nature, researchers led by Tony Wyss-Coray at Stanford University turn this idea on its head. They found that in young healthy mice, the blood-brain barrier allows in large quantities of endogenous plasma protein via receptor-mediated transport. This physiological uptake dwindles with age, even as nonspecific passage of large molecules rises. Overall, these changes result in less protein influx to the aging brain, rather than more. Curiously, inhibiting a single alkaline phosphatase can restore receptor-mediated uptake to old brains, the authors found.
- In young mice, plasma proteins pass readily into the brain.
- With age, this receptor-mediated uptake slows.
- Inhibiting an alkaline phosphatase can restore it.
“It’s really startling that plasma proteins get into the brain freely. We didn’t know that before,” said Costantino Iadecola at Weill Cornell Medical College, New York. “This paper will open a new chapter in blood-brain barrier biology.”
Others were intrigued by implications for disease. “It will be crucial to understand how the age-related transition in protein entry into the brain affects neural-circuit function, and whether this has a role in age-related cognitive decline,” Roeben Munji and Richard Daneman at the University of California, San Diego, wrote in an accompanying Nature editorial. The findings may also affect drug delivery, since several companies are developing strategies for ferrying drugs into the brain through the transferrin receptor. An age-related drop in efficiency could hinder this.
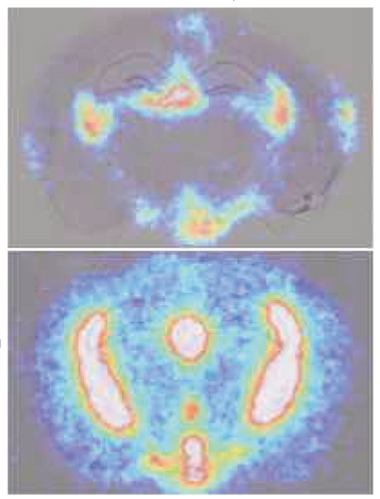
Free Entry. Radiolabeled plasma proteins (bottom) pass easily into the young mouse brain, while antibodies (top) are locked out. [Courtesy of Yang et al., Nature.]
Most studies of blood-brain barrier integrity infuse exogenous solutes such as dextran into the blood. Wyss-Coray and colleagues instead wanted to examine what happens with endogenous proteins. To do this, first author Andrew Yang collected blood from young healthy mice, depleted highly abundant proteins such as albumin and antibodies, and radiolabeled the remaining proteins. Twenty hours after infusing these proteins back into mice, the researchers found a strong signal in the brain, indicating high uptake. Entry was not indiscriminate for all proteins, however; in control experiments, very little labeled IgG entered the brain (see image at right).
The scientists were surprised by the amount of protein that got in. “At first we didn’t believe it,” Wyss-Coray said. To confirm, they labeled the plasma proteome in several other ways, including with fluorescent tags of varying charges and with chemical linkers such as biotin. In every case, they saw the same high brain entry. Fluorescently labeled plasma proteins appeared as bright spots inside brain endothelial cells, pericytes, neurons, and microglia (see image below). Fluorescence showed up in choroid plexus, indicating that proteins also cross the blood-cerebrospinal fluid barrier. In fact, “barrier” may be the wrong word for the blood-brain boundary, Wyss-Coray suggested. “It’s actually a very sophisticated filter of blood proteins,” he told Alzforum.

Vascular Highway. Fluorescently labeled plasma proteins show up as dots inside individual endothelial cells, indicating uptake. [Courtesy of Yang et al., Nature.]
Who let these proteins in? To identify the transporters, the researchers isolated endothelial cells four hours after injecting labeled plasma into the bloodstream. They sorted these cells by fluorescence, separating those with high and low uptake, and correlated that difference with gene expression via single-cell RNA sequencing of 745 cells. They found several expected genes associated with enhanced protein uptake, including the transferrin receptor, the amino acid transporter SLC3A2, the fatty acid transporter MFSD2A, and the lipoprotein transporter APOE.
However, the few genes associated with reduced brain uptake had not been known to have that function before, Wyss-Coray noted. Chief among these was the alkaline phosphatase ALPL, also known as tissue nonspecific alkaline phosphatase. This protein promotes bone mineralization (Murshed et al., 2005).
The transcriptomic analysis revealed a gradient along the vasculature, changing from sparse transporters in arterioles to venules riddled with transporters (see image below). It makes sense for venules to do most of the taking up, because blood pressure is lowest there, Wyss-Coray said. Most immune cells also enter the brain from venules.
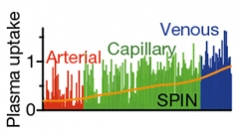
The Veins Have It. Endothelial gene expression shifts from allowing little protein uptake in arteries to robust uptake in veins. [Courtesy of Yang et al., Nature.]
“Just five years ago, we wouldn’t have been able to do this study, because the single-cell transcriptomic technology wasn’t there,” Wyss-Coray told Alzforum.
What happens with age? In 2-year-old mice, the pattern of uptake shifted. Entry of specific proteins dropped by half, while nonspecific influx of IgG rose sixfold. Examining a previously derived dataset of gene expression, the authors found that most of the genes that support specific protein uptake get expressed less with age. Meanwhile, genes involved in nonspecific transcytosis, such as the caveolar gene CAV1, go up (Yousef et al., 2019). The authors confirmed these expression changes by measuring the amount of the corresponding proteins in aged endothelium.
Likewise, when they looked at blood vessels from aged brain under a microscope, they found half as many clathrin-coated vesicles, which mediate receptor transport, but six times as many nonspecific caveolar vesicles, compared with the numbers in young mice. This again supports a shift from specific to nonspecific transcytosis with aging.

Old Pipes Lime Up. Blood vessels in old mice (bottom) develop calcium nodules (white, arrowheads) while young vessels (top) do not. [Courtesy of Yang et al., Nature.]
Part of the reason may be a loss of pericytes. These little cells surround blood vessels, and induce expression of transferrin, MFSD2A, and other transport genes. They die off as the brain ages (Feb 2018 news). Yang and colleagues correlated pericyte loss with expression changes in old mice, and they found calcium nodules in brain blood vessels in these old mice (see image at right). Notably, transgenic mice lacking pericytes develop calcifications (Keller et al., 2013).
One culprit may be the calcifying phosphatase ALPL. The authors found that its expression shoots up threefold in vasculature of aged mice. Because this phosphatase contributes to cardiac calcification, several companies are developing ALPL inhibitors. Yang and colleagues administered one to aged mice. It restored receptor-mediated transferrin uptake to the levels seen in young animals.
Possibly, such inhibitors could boost “brain shuttle” strategies that sneak therapeutics into aged brain via the transferrin receptor, Wyss-Coray suggested (Jan 2018 news; May 2020 news). “Now that the first transferrin receptor-based shuttles are in the clinic, this paper contributes to the understanding of how BBB shuttling mechanisms may change with age and reinforces the importance of continued research in humans to develop new and more efficient approaches for treating elderly patient populations,” agreed Jens Niewoehner at Roche (see RO7126209).
In future work, the scientists will identify specific proteins that pass into young brains, and explore how they change in mouse models of amyloidosis. Alterations in the blood-brain barrier have been linked to cognitive decline and seizures in AD (Jan 2019 news; Dec 2019 news).
“Numerous AD risk genes are involved in both receptor-mediated endocytosis and nonspecific uptake, suggesting that they could also contribute to AD pathogenesis via altered blood-brain barrier transcytosis,” Li-Huei Tsai, Joel Blanchard, and Adele Bubnys at the Massachusetts Institute of Technology, Cambridge wrote to Alzforum (full comment below).—Madolyn Bowman Rogers
References
News Citations
- Loss of Pericytes Wreaks Havoc in Mouse Brain
- Antibody Shuttle Rouses Anti-Aβ Response in Brain without Waking the Periphery
- Molecular Transport Vehicle Shuttles Therapies into Brain
- Absent Aβ, Blood-Brain Barrier Breakdown Predicts Cognitive Impairment
- Does a Breached Blood-Brain Barrier Cause Seizures in AD?
Therapeutics Citations
Paper Citations
- Murshed M, Harmey D, Millán JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005 May 1;19(9):1093-104. Epub 2005 Apr 15 PubMed.
- Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, Zandstra J, Berber E, Lehallier B, Mathur V, Nair RV, Bonanno LN, Yang AC, Peterson T, Hadeiba H, Merkel T, Körbelin J, Schwaninger M, Buckwalter MS, Quake SR, Butcher EC, Wyss-Coray T. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019 Jun;25(6):988-1000. Epub 2019 May 13 PubMed.
- Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JE, Rushing EJ, Hugelshofer M, Wurnig MC, Kaech A, Reimann R, Lohmann K, Dobričić V, Carracedo A, Petrović I, Miyasaki JM, Abakumova I, Mäe MA, Raschperger E, Zatz M, Zschiedrich K, Klepper J, Spiteri E, Prieto JM, Navas I, Preuss M, Dering C, Janković M, Paucar M, Svenningsson P, Saliminejad K, Khorshid HR, Novaković I, Aguzzi A, Boss A, Le Ber I, Defer G, Hannequin D, Kostić VS, Campion D, Geschwind DH, Coppola G, Betsholtz C, Klein C, Oliveira JR. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013 Sep;45(9):1077-82. Epub 2013 Aug 4 PubMed.
Further Reading
News
- Even Without Amyloid, ApoE4 Weakens Blood-Brain Barrier, Cognition
- Ruffles and Sphincters Control the Spigot of Fresh Blood in the Brain
- Scientists Discover a Common Distress Signal in the Blood-Brain Barrier
- VCAM1: Gateway to the Aging Brain?
- Focused Ultrasound Breaches Blood-Brain Barrier in People with Alzheimer’s
- Human Blood-Brain Barrier Model Blames Pericytes for CAA
Primary Papers
- Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, Vest RT, Chaney A, Lehallier B, Olsson N, du Bois H, Hsieh R, Cropper HC, Berdnik D, Li L, Wang EY, Traber GM, Bertozzi CR, Luo J, Snyder MP, Elias JE, Quake SR, James ML, Wyss-Coray T. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020 Jul 1; PubMed.
- Munji RN, Daneman R. Young brains welcome protein. Nature. 2020 Jul 1.
Annotate
To make an annotation you must Login or Register.

Comments
Picower Institute of MIT
Icahn School of Medicine at Mt. Sinai
MIT
In this paper, Yang et al. present a novel method of labeling whole panels of plasma proteins to measure BBB permeability in the aging mouse. By labeling endogenous blood-plasma peptides, they were able to specifically measure physiological rates of plasma uptake into cells of the brain parenchyma. They then coupled this powerful technique with single-cell RNA sequencing to identify the unique transcriptional signature of cells with high plasma uptake.
Interestingly, plasma uptake varied by vessel segment, with venous endothelial cells having the highest rates of plasma uptake, and this rate increased with age. By cross-referencing their list of plasma-uptake-associated genes with an existing dataset of the aging brain endothelial cell transcriptome, Yang et al. further identified an age-related shift from receptor-mediated transport to nonspecific calveolar transcytosis that appears to underlie the increased permeability of the aged BBB.
Although the authors limited their study to C57BL/6 mice that undergo normal aging, many of the mechanisms that underlie age-related changes in BBB permeability are likely to be relevant to Alzheimer’s disease, as well. The authors note a correlation between loss of pericyte coverage and increased barrier plasma transport, accompanied by decreased expression of pericyte-induced genes in the endothelium. These findings are consistent with a recent report that pericyte degeneration contributes to age-related BBB breakdown and is particularly accelerated in individuals with APOE4 (Nation et al., 2019; Montagne et al., 2020).
Numerous AD risk genes are involved in both receptor-mediated endocytosis and nonspecific uptake, suggesting that they could also contribute to AD pathogenesis via altered BBB transcytosis. We further note that a shift from ligand-specific transport to calveolin-mediated transcytosis may make the BBB more amenable to peripheral immune cell infiltration, an area of increasing interest in the AD field.
This paper raises interesting questions and provides a powerful plasma uptake-transcriptomics approach toward identifying new mechanisms and therapies to combat BBB breakdown in aging and AD. The study also adds to the evidence in support of a vascular AD hypothesis that implicates both endothelial cell barrier function breakdown and pericytes’ unique contributions to the neurovascular unit.
References:
Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TL, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019 Feb;25(2):270-276. Epub 2019 Jan 14 PubMed.
Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TL, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020 May;581(7806):71-76. Epub 2020 Apr 29 PubMed.
Denali Therapeutics
Denali Therapeutics & the University of Minnesota
This new work from the Wyss-Coray lab is an interesting study with impressive depth. Yang et al. employed a novel experimental design that relied upon the collection of mouse plasma and labeling of potentially the entire plasma proteome with either a radiolabel or 844 Da fluorophore for intravenous injection into either naive young (3 month) or aged (20-24 month) mice, and evaluation of brain uptake. Unsurprisingly, brain autoradiography and imaging of labeled plasma protein, as well as labeled IgG alone, showed prominent preferential uptake at ventricular and extra-ventricular CSF contacting sites, circumventricular organs, and perivascular spaces, consistent with emerging concepts of plasma protein uptake at the blood-CSF barriers and subsequent biodistribution along perivascular pathways into the brain from the CSF (Abbott et al., 2018).
Yang et al. logically focused on labeled protein internalization events at the level of the brain vasculature, interpreting brain uptake of whole plasma as well as individual proteins such as transferrin (Tf) primarily as the result of BBB transport. However, their inability to truly isolate the role of BBB transport from brain uptake due to blood-CSF barrier transport and other biodistribution processes suggests a possible caveat to some of the conclusions. Another limitation is the paper’s reliance on quantification of fluorescence imaging, radioactivity, and scRNAseq data to assess biodistribution and protein expression (e.g. Tf receptor) without additional methods such as the absolute quantification of protein levels (e.g. using lysates from tissue and the cerebrovasculature; Kariolis et al., 2020). Furthermore, the plasma labeling process may have influenced the activity, binding, and trafficking of certain plasma proteins in a manner that would seem hard to appreciate with the methods employed.
Nevertheless, the primary novelty of the study is the highly interesting observation of differential plasma protein brain uptake across young and aged mice and a proposed physiological mechanism involving its regulation by the alkaline phosphatase ALPL. These new findings highlight an area of longstanding debate in the BBB field relating to just how aging and disease may impact barrier function.
Several specific results warrant further comment. One of the most interesting findings reported by Yang et al. concerns a proposed shift in the character of BBB transport across brain endothelial cells in young versus old mice and a potentially new strategy to manipulate it. They report a loss of clathrin adaptor activity in brain endothelial cells as well as a decline in clathrin vesicles in isolated microvessels with age. Conversely, caveolar vesicles were found to significantly increase with age in isolated microvessels (and levels of MFSD2A, an inhibitor of caveolae-mediated transcytosis (Andreone et al. 2017), were shown to decrease with age as measured by quantification of MFSD2A staining from imaging of the vasculature. Filtering of their data allowed Yang et al. to zero in on Alpl, a gene correlated with inhibited plasma uptake that they also found to be upregulated in aged mouse brain endothelial cells. Alpl encodes the brain endothelial cell surface alkaline phosphatase ALPL.
Yang et al. took advantage of a previously identified, commercially available small molecule inhibitor of ALPL to show that ALPL inhibition in aged mice was capable of significantly increasing brain uptake of circulating Tf and labeled plasma and, to a lesser degree, a high affinity TfR antibody. If this finding proves robust and, importantly, applicable across species beyond the mouse, it may prove to be quite important. Yang et al.’s finding that TfR expression is reduced at the BBB with age stands in contrast to several previous studies. Recently, Frédéric Calon’s group compared 12 versus 18 month-old mice and observed no age-dependent TfR expression differences in isolated brain microvessels between wild-type mice or the 3xTg Alzheimer’s disease mouse model (Bourassa et al., 2019). Bourassa et al. further demonstrated no change in TfR-mediated transport capacity for a systemically administered bivalent, high- affinity TfR mAb across an age range of 12-22 months in both wild-type and 3xTg-AD model mice. This is consistent with an earlier report showing no change in TfR-mediated brain uptake of a monovalent, low-affinity TfR antibody across an age range of 5 to 10-13 months in both wild-type and PS2-APP model mice (Bien-Ly et al., 2015). Most importantly, human AD and age-matched controls do not differ with respect to TfR expression measured in cortical tissue lysate (Bien-Ly et al., 2015; Bourassa et al., 2019).
Taken together, these previous studies demonstrate a lack of change in TfR protein levels with age and disease state in mouse brain and microvessels, as well as a lack of change in TfR protein levels between AD and control brain and microvessels from human samples. The discrepancy between this prior mouse work and Yang et al. with respect to cerebrovascular TfR expression across the mouse lifespan merits further study. It is potentially attributable to methodological differences and possibly also the larger age window (3 vs. 20-24 months) used in the present study. It will also be important to carefully evaluate whether other receptor-mediated transcytosis targets may be altered in aged mice, whether there are brain regional differences in these changes and, importantly, whether the gene expression changes observed in mice are translatable to aged humans.
At the least, the Yang et al. findings provide interesting new hypotheses for how aging can impact the BBB, but ultimate translatability to the clinical setting and the impact on brain transport capacity remains unclear.
References:
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system?. Acta Neuropathol. 2018 Mar;135(3):387-407. Epub 2018 Feb 10 PubMed.
Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, Giese T, Assimon VA, Chen X, Zhang Y, Solanoy H, Jenkins K, Sanchez PE, Kane L, Miyamoto T, Chew KS, Pizzo ME, Liang N, Calvert ME, DeVos SL, Baskaran S, Hall S, Sweeney ZK, Thorne RG, Watts RJ, Dennis MS, Silverman AP, Zuchero YJ. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. 2020 May 27;12(545) PubMed.
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron. 2017 May 3;94(3):581-594.e5. Epub 2017 Apr 13 PubMed.
Bourassa P, Alata W, Tremblay C, Paris-Robidas S, Calon F. Transferrin Receptor-Mediated Uptake at the Blood-Brain Barrier Is Not Impaired by Alzheimer's Disease Neuropathology. Mol Pharm. 2019 Feb 4;16(2):583-594. Epub 2019 Jan 17 PubMed.
Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, Shihadeh V, Ulufatu S, Foreman O, Lu Y, DeVoss J, van der Brug M, Watts RJ. Lack of Widespread BBB Disruption in Alzheimer's Disease Models: Focus on Therapeutic Antibodies. Neuron. 2015 Oct 21;88(2):289-97. PubMed.
University of Edinburgh
This elegantly designed study sounds like a mini- “thunderclap” or “revolution” for the field of blood-brain barrier (BBB) research. It is accepted that the BBB becomes more permeable as we age (Montagne et al., 2015). However, this article suggests that things might be less straightforward, which will likely impact our current way of thinking of how the BBB functions and dysfunctions in health and disease. Yang and colleagues were able to label endogenous mouse plasma proteins and study their interactions with the BBB in young and aged mice. The authors found that blood-derived proteins can enter the young brain via receptor-mediated transcytosis (RMT) as receptors and components of clathrin-coated pits are highly expressed at the endothelium. At an older age, this process actually decreases and shifts towards a non-RMT process, meaning a non-specific transport across the BBB which involves caveolar vesicles. It tells us that blood-derived molecules can reach the brain parenchyma in youth and potentially affect or harm neuronal and cognitive functions prematurely.
The authors also found that age-related pericyte degeneration promotes the downstream shift in endothelial transport from ligand-specific RMT to non-specific caveolar transcytosis. These interesting results are in agreement with our clinical findings showing that BBB breakdown occurs during normal aging using dynamic contrast-enhanced magnetic resonance imaging. Importantly, our MRI measures correlate very well with the levels of soluble platelet-derived growth factor receptor-β in the cerebrospinal fluid which reflect pericyte injury/loss in the central nervous system (Montagne et al., 2015; Nation et al., 2019; Montagne et al., 2020). Furthermore, the results from Yang et al. along with ours seem to suggest that vascular dysfunction and pericyte degeneration do not depend on, but might be exacerbated by and in turn exacerbate, amyloid-β build-up in Alzheimer’s brains.
Two important things are missing from this study and follow-up studies will likely address them in the near future. First, identification of the proteins that are entering the young and older brain, and second, the concept of zonation, which is not only restricted to the arterio-capillary-venous axis (Vanlandewijck et al., 2018) but also across brain regions. It will be interesting to investigate whether this age-dependent shift in transcytosis is similar across all brain regions or whether regional differences exist. For instance, we know that the medial temporal lobe, which includes the hippocampus, is among the first regions being leaky during normal aging and worsening in people at risk for AD. Also, a preclinical study in pericyte-deficient mice found that early fibrin(ogen) extravascular deposits in white matter trigger pericytes’ and oligodendrocytes’ death by autophagy, ultimately leading to axon and myelin damage followed by neuronal and cognitive dysfunctions (Montagne et al., 2018). Altogether, these studies seem to point to a regional susceptibility where this reported shift in transcytosis could be explored.
References:
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015 Jan 21;85(2):296-302. PubMed.
Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TL, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020 May;581(7806):71-76. Epub 2020 Apr 29 PubMed.
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018 Mar;24(3):326-337. Epub 2018 Feb 5 PubMed. RETRACTED
Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TL, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019 Feb;25(2):270-276. Epub 2019 Jan 14 PubMed.
Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018 Feb 14; PubMed.
The University of Queensland
I find the work of Tony Wyss-Coray’s laboratory absolutely fascinating. It reveals a differentiated look at the BBB and how it changes under conditions of physiological aging. It is very relevant for our work.
Many labs, including ours, aim to use therapeutic ultrasound in conjunction with intravenously injected microbubbles to open the BBB in a controlled manner as a treatment modality for neurodegenerative diseases such as Alzheimer’s (Lipsman et al., 2018; Jordao et al., 2010; Choi et al., 2011; Leinenga and Götz, 2015). Interestingly, such a strategy achieved, in the absence of a therapeutic agent, a massive reduction of amyloid levels and amyloid plaque load, and a restoration of cognitive functions (Leinenga and Götz, 2015).
As an underlying mechanism, microglial activation and uptake of amyloid into lysosomes was revealed. The effect was thought to be brought about by a blood-borne factor that enters the brain and activates the dormant microglia.
Incidentally, BBB opening can be histologically visualized by intravenously injecting Evans Blue, which is normally excluded from the brain. To enter the brain, Evans Blue uses a blood-borne protein, albumin, as carrier. Albumin is not the only protein entering, but then again, there are effective mechanisms in place of expelling from the brain what has entered. Also, after ultrasound exposure the BBB closes again after several hours, i.e., opening is only transient. I could not agree more with what Costantino Iadecola wrote, that this, that Tony’s paper, will open a new chapter in BBB biology.
References:
Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018 Jul 25;9(1):2336. PubMed.
Leinenga G, Götz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci Transl Med. 2015 Mar 11;7(278):278ra33. PubMed.
Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16539-44. Epub 2011 Sep 19 PubMed.
Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One. 2010;5(5):e10549. PubMed.
University of Arizona College of Medicine and Pharmacy
Excellent work by Tony Wyss-Coray's laboratory. It advances the field with impressive and exciting observations and hypotheses that confirm what several of our BBB colleagues have been discussing in recent years. I agree with Axel Montagne that this work will likely impact our current way of thinking of how BBB functions and dysfunctions in health and disease. So very good to see the excitement, as evidenced by the in-depth comments.
View all comments by Tom DavisMake a Comment
To make a comment you must login or register.