Synuclein Assay Passes the Sniff Test—What of Other Seeds?
Quick Links
Seed amplification assays, those PCR-like reactions for toxic misfolded proteins, are starting to look pretty good. In the May Lancet Neurology, scientists led by Andrew Siderowf, University of Pennsylvania, Philadelphia, and Luis Concha-Marambio, Amprion, San Diego, reported the largest SAA CSF study to date, of more than 1,100 samples from the Parkinson’s Progression Markers Initiative. Their α-synuclein seeding assay detected sporadic PD with high sensitivity and specificity.
- Seed amplification assays can detect teeny amounts of misfolded protein.
- A synuclein SAA pegs sporadic Parkinson's disease with high sensitivity.
- Its sensitivity is lower in familial PD, or if sense of smell is normal.
- The assay also detects mixed pathology in people with Alzheimer's.
But there were some twists, and not necessarily of the molecular kind. The best detection rate, 99 percent, came from people with a poor sense of smell, a common though not universal symptom of PD. Among carriers of mutations in the LRRK2 gene, only 67 percent tested positive.
“The test is very specific, but the other interesting thing is that it reveals heterogeneity in the disease,” Siderowf told Alzforum. That heterogeneity could be important when planning, or interpreting, clinical trials. For example, 86 percent of people who had symptoms of prodromal PD tested positive in the seeding amplification assay (SAA), while only 8 percent of asymptomatic LRRK2 and GBA mutation carriers did. “These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined, at-risk cohorts,” wrote the authors.
“The good news is that we have entered a new era of biomarker and treatment development for Parkinson’s disease,” wrote Daniela Berg, Christian Albrechts-University, Kiel, and Christine Klein, University of Lübeck, both in Germany, in a comment to Lancet Neurology. “The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing a seed amplification assay (SAA), is a seminal development,” they added.
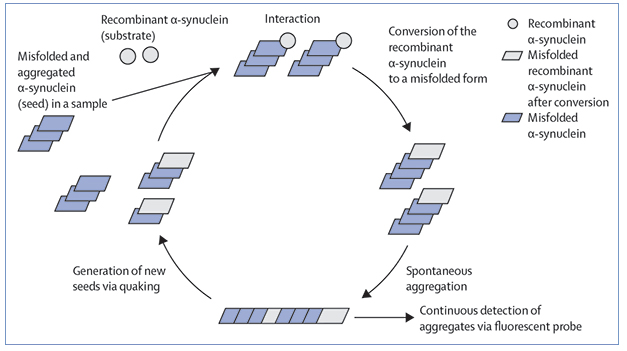
Synuclein SAA. In Real Time Quaking-Induced Conversion (RT-QuIC) assays, fibrils of α-synuclein coax recombinant forms to misfold and extend the fibril. Shaking fragments the fibrils, setting the whole process in motion again. [Courtesy of Inga Zerr, Lancet Neurology, 2021].
As was evident at AD/PD 2023 in Gothenburg, Sweden, scientists are turning to SAAs for other neurodegenerative diseases, as well. Scientists led by Oskar Hansson, Lund University, Sweden, are looking to SAAs to identify α-synuclein pathology in people with AD. Lewy bodies are the most common comorbidity in people with AD under age 85, and Hansson thinks it is important to understand how these inclusions contribute to clinical symptoms, especially since they are not targeted by approved anti-amyloid immunotherapies or by promising anti-tau therapies (Apr 2023 conference news).
Others are trying to optimize assays to detect tau seeds in the CSF, which has proven a tough nut to crack. Bryan Frey from AbbVie, Ludwigshafen am Rhein, Germany, reported that he sees higher signals in samples from amyloid-positive donors than from amyloid-negative ones, although the signal overlaps and does not correlate with cognition or other tau markers. In short, while researchers are making progress on these tests, questions remain.
Seed amplification assays are based on the principle of templated misfolding. A protein fibril binds a native monomer and stabilizes it in a toxic conformation, whereby it joins the end of the growing fibril. In practice, the tests sound simple enough—spike a solution of protein monomers with a biological sample containing fibrils and wait for the seeds to do their thing (see image above). In practice, it is tricky, requiring the growing fibrils be split apart to create new seeds to amplify the process. Success depends on the number of such cycles and the quality of the monomers used, since many of the proteins of interest tend to self-aggregate, yielding false positives.
Fragmentation can be done by physically shaking or sonicating the reaction mixture, as in Real Time Quaking-Induced Conversion, or RT-QuIC, developed by Byron Caughey’s lab at the Rocky Mountain Laboratories, National Institutes of Health, Hamilton, Montana, or the Protein-Misfolding Cyclic-Amplification (PMCA) method developed by Claudio Soto at the University of Texas Medical School, Houston (Mar 2014 news). Soto is a senior author on the Lancet Neurology paper. Those tests were initially developed to detect prion proteins; they are used to diagnose Creutzfeldt-Jakob disease in people and prion diseases in animals.
SAAs are now widely used by manly labs, and those for α-synuclein, at least, seem to work quite well. A prior analysis of 80 PPMI samples by Soto, Caughey, and Un Jung Kang’s group at New York University Grossman School of Medicine found that three different versions performed equally, identifying clinically confirmed PD cases with high sensitivity and specificity (Russo et al., 2021). Siderowf and colleagues expanded that analysis to 1,123 PPMI samples. “It’s a nice extension of what a number of other studies have found with smaller numbers of patients in multiple cohorts,” Caughey told Alzforum.
Previously, Caughey and Hansson had reported that the RT-QuIC SAA of CSF samples from the BioFinder cohort in Sweden identified people with Lewy body disorders with 95 percent sensitivity, while in collaboration with Kang, Caughey reported that the assay detected α-synuclein seeds in 102 PPMI CSF samples with high sensitivity (Hall et al., 2022; Orrù et al., 2021). Caughey, Piero Parchi at the University of Bologna, Italy, and colleagues found that RT-QuIC accurately identified synucleinopathy among 439 clinically characterized volunteers who had donated samples to the Institute of Neurological Sciences there (Rossi et al., 2020). That cohort included people with isolated REM sleep behavior disorder (IRBD) and pure autonomic failure, two clinical symptoms that commonly precede PD. Likewise, scientists led by Alison Green at the University of Edinburgh and Alex Iranzo at the Hospital Clínic de Barcelona found that among 52 people with IRBD, 32 developed PD or dementia with Lewy bodies within seven years, and CSF from 31 of those had tested positive for α-synuclein seeds at baseline, suggesting that the assay detects prodromal disease (Iranzo et al., 2021).

Better Than a Smell Test? People with sporadic or familial PD who have a dopaminergic deficit (y axis) and test positive on the α-synuclein SAA (closed circles) don’t always have a poor sense of smell (x axis). Horizontal and vertical bars represent cutoffs for dopamine transporter SPECT imaging and the UPSIT smell test, respectively. [Courtesy of Andrew Siderowf, Lancet Neurology, 2023.]
Siderowf and colleagues came to a similar conclusion. Among the PPMI volunteers they tested, 545 had been diagnosed with PD. Of them, 373 had sporadic disease, 123 carried a LRRK2 Gly2019Ser mutation, and 49 an Asn409Ser mutation in their GBA gene. Another 54 volunteers had parkinsonism, but appeared normal on brain imaging scans for dopaminergic deficit, while 51 had evidence of prodromal PD, be that IRBD or hyposmia, i.e., loss of smell. They also tested CSF from 310 asymptomatic carriers of LRRK2 or GBA mutations and from 163 healthy controls.
The SAA detected sporadic PD with a sensitivity of 88 percent, while the specificity for ruling out normal controls was 96 percent. Among sporadic cases who performed below the 15th percentile on the University of Pennsylvania Smell Identification Test, the sensitivity was even higher, at 99 percent (see image above). About 90 percent of people with PD have hyposmia. Among PD patients without hyposmia, 78 percent tested positive.
The sensitivity was lower in other subgroups, also. In LRRK2 PD, only 67 percent tested positive, falling to 35 percent in those who passed the scratch and sniff test. Why these numbers are low remains to be investigated. One possibility is that LRRK2 carriers who don’t test positive may have a different type of Parkinsonism, said Siderowf. Indeed, some LRRK2 PD cases have been reported to have no Lewy body pathology (Kalia et al., 2015). In the PPMI cohort, 15 of the PD volunteers had died and their brains were autopsied. One had no Lewy bodies or Lewy neurites, but had lost neurons in the substantia nigra. This person turned out to have the LRRK2 mutation and normal sense of smell.
Why the SAA test does better in those with hyposmia needs investigation, too. “In prodromal participants, we looked hard at all the clinical features—cognitive, autonomic, affective disorder, neuropsychology, etc., but none correlated with a positive SAA, bar olfactory deficit,” said Siderowf. It has been hypothesized that PD starts in the olfactory bulb, and Siderowf believes the SAA test presents an opportunity to test that further (Braak et al., 2003; Jan 2010 news; Dec 2015 conference news).
Can the SAA predict who will develop a Lewy body disorder? Of the 310 asymptomatic carriers, 25 tested positive, suggesting these might be in the early stages of Parkinson’s. Future analysis may tell, since the PPMI is a 10-year study, and only the baseline SAA data are reported in this paper. In the meantime, the current data hint that the test is prognostic. Among 51 volunteers who had hyposmia or IRBD, 44 tested positive on the SAA test but only 13 of those had SPECT-DAT imaging suggesting dopaminergic deficit. This suggests that the SAA picks up synuclein pathology first. “DAT scans typically detect deficits three years before symptoms, but the SAA test could push that back another three to five years,” suggested Siderowf. “Among GBA mutation carriers, about 7 to 8 percent were positive on SAA but not on DAT scans, so it’s possible that in these cases the SAA turns positive many years before the patient develops PD,” he said.
Hansson thought this was very exciting. “I found this extremely encouraging, especially for preclinical trials,” he told Alzforum. Still, Hansson would like to see more studies of normal people to see how many have Lewy bodies according to this assay. Indeed, Iranzo and colleagues had reported that four of 40 healthy controls in the Barcelona cohort tested SAA-positive, and 10 years later three were still healthy, while the fourth, who had been admitted to the hospital with an infection, was unable to complete the study. “The probability of some participants having a subclinical α-synucleinopathy that did not evolve into Parkinson’s disease or dementia with Lewy bodies within the observation period cannot be excluded and requires further investigation,” wrote Inga Zerr, University Medical Center, Georg-August University, Göttingen, Germany, in a Lancet Neurology editorial at the time.
Siderowf acknowledged the possibility. Indeed, in PPMI, about 4 percent of healthy controls also tested positive. “Is this random variation, false positives, or is there a number of people in the general population who do have misfolded seeds of α-synuclein in their CSF who do not, or will never, get PD?” he asked. Such resilience might hold the key to novel therapeutics, he suggested.
SAAs for Other Diseases
These assays have potential as diagnostics for other disorders, as well. They would be particularly helpful when no imaging or fluid marker is available, such as in TDP-43-based diseases, or mixed pathologies. On the latter, Hansson showed some preliminary data from almost 2,000 volunteers in the Swedish BioFinder cohort. It suggested that the α-synuclein seeding assay, together with AD biomarker analysis, detects comorbidities.
He reported that among cognitively unimpaired volunteers, about 12 percent had AD, 6 percent had Lewy body disease, while about 2 percent had both. In cognitively impaired people, the numbers for AD, LBD, and AD/LBD jumped to 42, 12, and 11 percent, respectively. Hansson promised to show more at the AAIC meeting next July. He believes these numbers will have important ramifications for clinical trials because both the Lewy bodies and tau pathology correlated equally well with memory loss in cognitively unimpaired volunteers. “I was quite surprised by this,” Hansson told the audience. He said longitudinal data suggests that having Lewy body pathology in AD is detrimental. “It will be important to better understand variability in response to therapies in trials to see what happens to these individuals,” he told Alzforum.
Frey’s talk in Gothenburg emphasized that it's still difficult to optimize SAAs for other proteins. Indeed, researchers in Marc Diamond’s lab, UT Southwestern Medical Center, Dallas, found no tau seeds in CSF from AD patients using biosensor cell lines (see Part 3 of this series) , but scientists in Caughey’s lab were able to detect seeds in frontotemporal dementia CSF using an RT-QuIC assay (Hitt et al., 2021; Saijo et al., 2020). Notably, the tau fibrils that form in AD and FTD are different. The former comprise tau with both 3- and 4-repeat units, whereas the latter is 4R tau only. Frey and colleagues at AbbVie tried to optimize an SAA test for the 3R/4R forms of tau found in AD.
By changing the substrate in the assay to a small fragment that includes the repeat domains and an elongated C-terminal tail, Frey struck pay dirt. In this system, AD CSF boosted fluorescence, an indication of fibrillization, by 5,000-fold over that seen with CSF from healthy controls. In a pilot study, the assay detected 40 percent more fluorescence from AD samples than from Aβ-negative, age-matched control CSF. However, there was considerable overlap between the 10 AD and five control samples. A larger study of 48 samples indicated a bigger group difference of 65 percent, but again, the overlap was substantial. Frey is not sure why. It could be that the assay needs to be optimized further, or it might be that controls also have tau seeds. Some scientists believe that fibrils of tau can begin to form in the brain with age, even without any amyloid present (Nov 2014 news).
Caughey noted that, in his hands, tau SAAs are less robust than α-synuclein assays. “We get more chatter from healthy controls, so while a lot of people have tau seeds in their CSF, the data are not quite as consistent,” he told Alzforum. “The presence of different types of pathological and non-pathological aggregates and co-pathologies is part of the landscape,” he said. Still, he says it’s early days and he thinks tau RT-QuIC assays will become helpful diagnostically. As for TDP-43, one recent paper reported that RT-QuIC detects seeds in the CSF of people who had ALS/FTD (Scialò et al., 2020).—Tom Fagan
References
News Citations
- First Hit on Aggregated Tau: Antisense Oligonucleotide Lowers Tangles
- Test Uses 'Seeding' to Detect Aβ Oligomers in Cerebrospinal Fluid
- Blunted Sense of Smell Parallels Pathology in AD, PD
- Lewy Pathology in DLB Spreads Fast, Maybe From the Nose
- Tau Chimeras Do Make Fibrils—and a Chaperone Rips Them Apart
- Scientists Propose a New Definition for Tau-Only Pathology
Paper Citations
- Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM, Holguin B, Hughson AG, LaFontant DE, Caspell-Garcia C, Coffey CS, Mollon J, Hutten SJ, Merchant K, Heym RG, Soto C, Caughey B, Kang UJ. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson's disease. Acta Neuropathol Commun. 2021 Nov 6;9(1):179. PubMed. Correction.
- Hall S, Orrù CD, Serrano GE, Galasko D, Hughson AG, Groveman BR, Adler CH, Beach TG, Caughey B, Hansson O. Performance of αSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol Commun. 2022 Jun 22;10(1):90. PubMed.
- Orrù CD, Ma TC, Hughson AG, Groveman BR, Srivastava A, Galasko D, Angers R, Downey P, Crawford K, Hutten SJ, Kang UJ, Caughey B. A rapid α-synuclein seed assay of Parkinson's disease CSF panel shows high diagnostic accuracy. Ann Clin Transl Neurol. 2021 Feb;8(2):374-384. Epub 2020 Dec 29 PubMed.
- Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orrù CD, Antelmi E, Mammana A, Hughson AG, Calandra-Buonaura G, Ladogana A, Plazzi G, Cortelli P, Caughey B, Parchi P. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020 Jul;140(1):49-62. Epub 2020 Apr 27 PubMed. Correction.
- Iranzo A, Fairfoul G, Ayudhaya AC, Serradell M, Gelpi E, Vilaseca I, Sanchez-Valle R, Gaig C, Santamaria J, Tolosa E, Riha RL, Green AJ. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021 Mar;20(3):203-212. PubMed.
- Kalia LV, Lang AE, Hazrati LN, Fujioka S, Wszolek ZK, Dickson DW, Ross OA, Van Deerlin VM, Trojanowski JQ, Hurtig HI, Alcalay RN, Marder KS, Clark LN, Gaig C, Tolosa E, Ruiz-Martínez J, Marti-Masso JF, Ferrer I, López de Munain A, Goldman SM, Schüle B, Langston JW, Aasly JO, Giordana MT, Bonifati V, Puschmann A, Canesi M, Pezzoli G, Maues De Paula A, Hasegawa K, Duyckaerts C, Brice A, Stoessl AJ, Marras C. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015 Jan;72(1):100-5. PubMed.
- Braak H, Del Tredici K, Rüb U, De Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197-211. PubMed.
- Hitt BD, Vaquer-Alicea J, Manon VA, Beaver JD, Kashmer OM, Garcia JN, Diamond MI. Ultrasensitive tau biosensor cells detect no seeding in Alzheimer's disease CSF. Acta Neuropathol Commun. 2021 May 26;9(1):99. PubMed.
- Saijo E, Metrick MA 2nd, Koga S, Parchi P, Litvan I, Spina S, Boxer A, Rojas JC, Galasko D, Kraus A, Rossi M, Newell K, Zanusso G, Grinberg LT, Seeley WW, Ghetti B, Dickson DW, Caughey B. 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. 2020 Jan;139(1):63-77. Epub 2019 Oct 16 PubMed.
- Scialò C, Tran TH, Salzano G, Novi G, Caponnetto C, Chiò A, Calvo A, Canosa A, Moda F, Caroppo P, Silani V, Ticozzi N, Ratti A, Borroni B, Benussi L, Ghidoni R, Furlanis G, Manganotti P, Senigagliesi B, Parisse P, Brasselet R, Buratti E, Legname G. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020;2(2):fcaa142. Epub 2020 Sep 14 PubMed.
Further Reading
Papers
- Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, Campbell KJ, Safar J, Galasko D, Caughey B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018 Feb 9;6(1):7. PubMed. Correction.
Primary Papers
- Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LM, Hutten SJ, Sherer T, Marek K, Soto C, Parkinson's Progression Markers Initiative. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023 May;22(5):407-417. PubMed.
- Berg D, Klein C. α-synuclein seed amplification and its uses in Parkinson's disease. Lancet Neurol. 2023 May;22(5):369-371. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.