Parkinson's Therapies Seek to Stem Progression
Quick Links
At the second biannual Advances in Alzheimer’s and Parkinson’s Therapies Focus Meeting (AAT-AD/PD), held virtually April 2 to 5, companies offered some news on investigational Parkinson’s therapies in Phase 1. One approach is targeted toward a common form of PD caused by mutations in the glucocerebrosidase gene that slow down glycosphingolipid flux. Biotech company Lysosomal Therapeutics in Cambridge, Massachusetts, developed a small-molecule activator of glucocerebrosidase, LTI-291, that unclogged the recycling pathway and appeared to benefit brain function in Phase 1b. Taking a different tack, San Diego-based Neuropore Therapies deploys antagonists of the toll-like receptor 2 (TLR2) in hopes of dampening harmful immune responses while boosting autophagy of misfolded proteins. This approach not only is broadly applicable to all forms of PD, but also could help with proteinopathies beyond α-synucleinopathies. The company has decided to develop its Phase 1 compound NPT520-34 for amyotrophic lateral sclerosis.
- Glucocerebrosidase activator greases lipid recycling in a genetic form of PD.
- In Phase 1b, the strategy normalized brain function.
- TLR2 antagonist targets both autophagy and inflammation.
Restoring Lipid Recycling
Up to 10 percent of Parkinson’s patients carry a mutation in the glucocerebrosidase (GBA) gene. These mutations heighten disease risk up to 15-fold, and cause a severe form of PD. GBA-PD progresses more rapidly than idiopathic disease and is more likely to lead to dementia. At AAT-AD/PD, Kees Been, who leads Lysosomal Therapeutics, discussed the company’s strategy for treating this form of PD. The glucocerebrosidase (GCase) enzyme catalyzes the breakdown of glucosylceramide to ceramide. This is a critical step in the recycling of membrane glycosphingolipids, which affect membrane properties. Mutations in GBA weaken GCase’s activity and slow the recycling. The researchers developed LTI-291, a small-molecule allosteric activator of GCase.
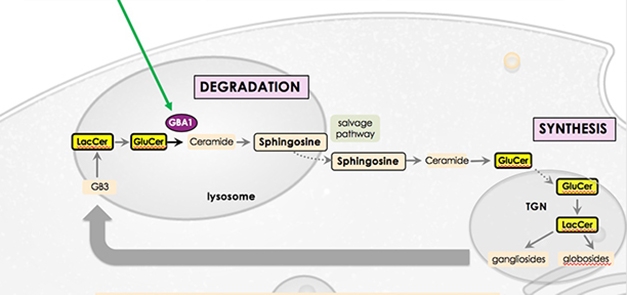
Controlling the Flow. GBA catalyzes a crucial step in the lysosomal breakdown of glycosphingolipids, allowing them to be synthesized anew and recycled to the plasma membrane. [Courtesy of Kees Been.]
In Phase 1 testing, about 1 percent of plasma LTI-291 entered the brain, achieving a concentration of 1 μM or better, based on the amount of free drug in CSF. This would be sufficient to double GCase activity, Been said. The compound has desirable drug properties, with a half-life of about 30 hours. It reached a steady state in the body after five or six days of dosing. About 98 percent of it became bound into membranes. This is necessary for it to work, since GCase resides in lysosomal membranes. That the fraction of free drug is small also helps reduce side effects, improving the compound’s safety, noted the company’s chief scientific officer, Peter Lansbury. “This drug is incredibly clean in toxicity assays. We have a huge therapeutic window,” Lansbury said.
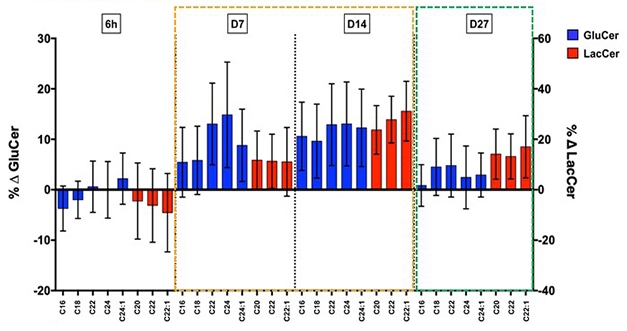
Clearing the Backlog. In participants treated with glucocerebrosidase activator LTI-291, synthesis of downstream lipids (blue and red bars) surged seven and 14 days later, before stabilizing after one month. [Courtesy of Kees Been.]
In a Phase 1b trial, 40 people with GBA-PD took either placebo or 10, 30, or 60 mg/day LTI-291 for 28 days. In treated patients, the researchers measured a transient surge in glycosphingolipids (GSLs) downstream of ceramide, indicating an accelerating recycling rate. The surge subsided as lipid recycling stabilized, presumably at a higher level. Lansbury compared this effect to the temporary rise in water level when a dam is released, before the current reaches a new, faster, equilibrium. The increase in GSLs was greater in people with more severe GBA mutations, whose recycling rate had been more suppressed before treatment.
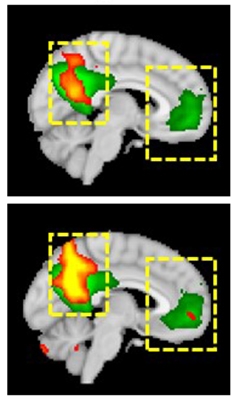
Improved Connectivity. Functional brain connectivity (yellow and red) in the default mode network improved (bottom) compared to baseline (top) in a Parkinson's patient taking LTI-291, getting closer to the activity seen in healthy controls (green). [Courtesy of Kees Been.]
The company also ran a Phase 1b imaging study of 14 people with GBA-PD. Participants took placebo, 10, or 60 mg/kg LTI-291 for 28 days. At the end of the study, brain function in treated participants had normalized on several measures. These included enhanced brain blood flow by arterial spin labeling, increased brain glucose metabolism by FDG PET, and better functional connectivity in the default mode network on fMRI scans. The effects were similar in all treated patients, but not statistically significant in this small cohort.
“We’re quite excited with these results,” Been said. Next, a Phase 2 study will test two doses plus placebo for one year in people with GBA-PD. The endpoints will be MDS-UPDRS score and cognitive change. Been said they are still deciding how many participants they need, but plan to power the trial to be able to detect a disease-modifying effect. He noted that this is the first allosteric activator of GCase to be tested in PD patients with a GBA1 mutation.
Dual Action on Inflammation and Autophagy
Another approach entering trials takes a different tack. At AAT-AD/PD, Douglas Bonhaus, who leads Neuropore Therapies, discussed the small molecule NPT520-34, an antagonist of TLR2. This immune receptor recognizes aggregated proteins and is expressed by both neurons and microglia. TLR2 expression rises as PD progresses, correlating with α-synuclein deposition (Dzamko et al., 2017).
In neurons, TLR2 turns down autophagy, which could contribute to the accumulation of misfolded proteins. In microglia, TLR2 signaling leads to activation of the NLRP3 inflammasome, which is associated with damaging responses to disease (Dec 2012 news; Nov 2019 news). Thus, TLR2 has a dual mechanism of action, the company’s Martin Gill, head of in vitro pharmacology, said at AAT-AD/PD.
The company identified NPT520-34 in a screen for compounds that boost autophagy. In cellular assays, the compound increased expression of the scaffolding protein LC3 about threefold, powering autophagy. In the L61 α-synucleinopathy mouse model, treatment with NPT520-34 dose-dependently lowered protein deposits. It also calmed neuroinflammation, with fewer activated microglia visible by TSPO imaging. NPT520-34 also preserved dopaminergic signaling and improved grip strength and walking, Gill said.
Notably, the compound stimulates clearance of other types of aggregate, as well. In the Line 41 mouse model of amyloidosis, it lowered amyloid plaques and TSPO signal. In SOD1-G93A mice, a model for amyotrophic lateral sclerosis, it curbed aggregates and inflammation in the spinal cord. When treatment began at symptom onset in these SOD1 mice, they survived for longer, 41 instead of 34 days.
In a first Phase 1 trial conducted in 2019 in 49 healthy volunteers, NPT520-34 appeared safe with repeated dosing of up to 500 mg per day, and had “well-behaved” pharmacokinetics. This maximum dose far exceeds the effective dose in animal models, equivalent to about 125 mg, Bonhaus noted. The most common adverse event was headache.
The company plans to evaluate this drug first in ALS, for which Neuropore Therapies received orphan drug designation in August 2019 (see press release). The researchers are currently doing a 28-day safety study in people with this disease. They hope to move forward with a three-month study that will include TSPO imaging, and then a six-month study of safety and efficacy.—Madolyn Bowman Rogers
References
News Citations
- Microglia and AD—Does the Inflammasome Drive Aβ Pathology?
- Microglia Inflammasome Stokes Tau Phosphorylation, Tangles
Research Models Citations
Paper Citations
- Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, Fu Y, Halliday GM. Toll-like receptor 2 is increased in neurons in Parkinson's disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017 Feb;133(2):303-319. Epub 2016 Nov 25 PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.