Donanemab Confirms: Clearing Plaques Slows Decline—By a Bit
Quick Links
It has been clear for a while that anti-amyloid antibodies can sweep plaque from the brain, but until now the question of whether this slows cognitive decline has remained hotly contended. Despite some positive signals from four such antibodies, the data have been messy and hard to interpret. At the 15th International Conference on Alzheimer’s and Parkinson’s Diseases, held virtually March 9–14, Mark Mintun of Eli Lilly & Company presented the cleanest data yet on this question. In a Phase 2 trial, the company’s anti-amyloid antibody donanemab met its primary endpoint. Participants did not get better. Even so, donanemab slowed their decline by an average of 32 percent on a combined cognitive and functional measure.
- In a Phase 2 trial, donanemab completely cleared plaque in two-thirds of participants.
- Their cognitive decline slowed by a third, meeting the primary endpoint.
- The treatment appeared to nudge down tangles, and worked best at low tangle loads.
Donanemab banished plaque from the brain in a majority of participants, while nudging down the rate of neurofibrillary tangle accumulation in the frontal cortex and other regions. The trial included several innovative elements, such as screening participants by tangle burden, using tau PET as a secondary outcome measure, and stopping dosing once amyloid was gone. The AD/PD presentation fleshed out previously announced topline data (Jan 2021 news). Full results were published March 13 in the New England Journal of Medicine (Mintun et al., 2021).
Most Alzheimer’s researchers welcomed the findings. “This was the first [disease-modifying] AD drug to meet a clinical endpoint in a Phase 2 trial,” noted Ron Petersen of the Mayo Clinic in Rochester, Minnesota. Michael Weiner of the University of California, San Francisco, found the broader implications encouraging. “In my view, together with data from other trials, this study strongly confirms the ‘amyloid hypothesis’ and demonstrates that treatments aimed at amyloid can slow cognitive decline and modify the progression of AD,” he wrote (full comments below).
At the same time, researchers emphasized that, as with other anti-amyloid immunotherapies, the cognitive benefit was small. “The donanemab story is the most encouraging news on the amyloid front, ever, but whether the effect size is clinically meaningful is questionable,” David Knopman at the Rochester Mayo clinic wrote to Alzforum (full comment below).
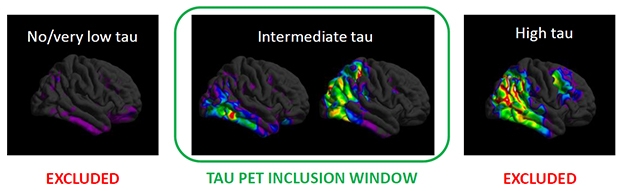
Screen By Path. People with few tangles (left) have too little cognitive decline to measure, while those with a heavy tangle burden (right) may have worsened beyond the reach of an anti-amyloid drug. Selecting for the just-right tangle load (middle) may have helped the donanemab trial succeed. [Courtesy of Eli Lilly.]
Plaque-Busting Power
Donanemab is unique among AD immunotherapies in that it targets a modified version of Aβ that has a pyroglutamate attached to the N terminus. This pathological form of Aβ is highly prone to aggregate, depositing in the core of all amyloid plaques, but is found nowhere else in the brain (Dec 2009 conference news; Nov 2010 conference news; Dec 2012 news). In Phase 1 trials, donanemab busted up plaques fast, in many cases clearing all deposits within six months (Aug 2018 conference news; Dec 2019 conference news).
However, even dramatic amyloid clearance has not translated into a clear cognitive benefit in past Phase 2 and 3 immunotherapy trials. In a company call with investors, Lilly’s chief scientific officer, Dan Skovronsky, said part of the problem in obtaining definitive cognitive results may arise from the heterogeneity of AD trial populations, with participants worsening clinically at different rates. To limit this variability, the researchers screened participants in their 18-month Phase 2 Trailblazer study using tau PET. They believed this might work because previous studies had shown that a person’s baseline tau PET signal predicted his or her speed of subsequent cognitive decline.
People with flortaucipir SUVRs below 1.1 were excluded from this trial, since studies have shown almost no cognitive decline in this group within the time span of this trial. Those with SUVRs above 1.46 were also excluded, as the researchers hypothesized that tangle pathology in their brains would be too advanced for an amyloid therapy to do them any good (see image above). Mintun estimates that 30 to 45 percent of people with early symptomatic AD fall into the intermediate tau range where anti-amyloid therapy might be effective.
The researchers ended up enrolling 257 people with this intermediate tangle burden at 56 sites across the United States and Canada. Participants were predominantly white, with an average age of 75, and about three-quarters carried an APOE4 allele. All had early symptomatic AD and were amyloid-positive by florbetapir PET scan, with a mean MMSE of 23.6. Mintun noted that this cognitive average is lower than for many other trials in early AD, where the cutoff for inclusion is often 24, and the average score higher. Selecting participants based on tangle pathology rather than clinical criteria may have allowed for a wider range in clinical status, he suggested. Because of cognitive reserve, people with similar levels of brain pathology often differ in the degree to which their function is preserved (Aug 2017 conference news). “Some patients who would be considered too impaired for inclusion in an early AD trial might still be at an early stage of pathology and respond to treatment,” Mintun noted.
Half the participants, 131 people, received donanemab, the other 126 placebo. Doses were titrated up rapidly for quick plaque clearance, with participants receiving 700 mg for the first three monthly infusions and 1,400 mg per month thereafter. Participants underwent florbetapir scans at weeks 24 and 52 to assess their progress. If their amyloid burden fell below 25 centiloids—the level in healthy young controls—their donanemab dose was lowered to 700. If it fell below 11 centiloids, or below 25 for two consecutive scans, they were switched to placebo.
Why stop dosing? Pyroglutamate-Aβ only occurs in plaques, so once the target is gone, there is no need for further treatment, Mintun said. Commentators applauded this limited course of treatment, given the expense and side effects of antibodies. “That patients could be withdrawn from the treatment is a remarkable prospect for broader use,” Knopman wrote. Petersen suggested it would make AD easier to manage chronically. “We may be able to lower amyloid levels, monitor the patients, and if the levels rise, re-dose. This would be akin to giving a booster immunization,” Petersen said.
In Trailblazer, the baseline amyloid burden was 108 centiloids in the active group and 101 in the placebo group. It stayed stable in the placebo group over the course of the study. In the active group, it dropped by an average of 85 centiloids. The bulk of the clearance came early, with an average drop of 68 centiloids by week 24. At that time, 40 percent of the treatment group were switched to placebo. This rose to 60 percent by 52 weeks and 68 percent by 76 weeks. In other words, two-thirds of participants were amyloid-negative by the end of the trial. Mintun noted that plaque clearance at 18 months was about twice that seen with aducanumab, in agreement with earlier trial results suggesting that donanemab clears plaque more aggressively than do other investigational antibodies.
In an ongoing open-label extension trial, participants who still have plaque, as well as previous placebo patients, will remain on donanemab treatment until they, too, become amyloid-negative. Researchers will follow all patients to assess how they fare over time.
Detecting a Cognitive Signal
Did this clearance translate into a better-functioning brain, though? To answer this, the researchers chose as their primary outcome measure the integrated Alzheimer’s Disease Rating Scale. Lilly had developed the iADRS by combining the ADAS-Cog13 with the ADCS-instrumental Activities of Daily Living scales (Wessels et al., 2015). In the Phase 3 solanezumab Expedition studies, this combined cognitive and functional scale yielded more consistent results than did the CDR-SB, Mintun said. Likewise, in the placebo arm of Trailblazer, the iADRS scores reflected a constant rate of decline, whereas the CDR-SB posted variability from timepoint to timepoint. Mintun said the CDR-SB has proven noisy and unreliable in other large AD studies, as well, for example giving one positive and one negative result in the aducanumab Phase 3 program. “We believe the iADRS is a more consistent and sensitive measure to detect treatment differences than other AD scales,” Mintun said.
In Trailblazer, participants started out with an average iADRS score of 106, with the placebo group declining 10.06 points by the end of the trial, and the active group by 6.86. The treatment groups started to diverge at 24 weeks, around the time plaque clearance became dramatic. The difference between groups reached statistical significance at 36 weeks and maintained that for every timepoint thereafter, with a final p value of 0.04. The one-third slowing of decline is modest. It would translate to a six-month delay in disease progression over the course of the 18-month trial, Mintun noted.
All secondary clinical measures trended in favor of donanemab, but only the ADAS-Cog13 reached significance at p=0.04, with an average slowing of 39 percent. On the CDR-SB, decline slowed only by 23 percent, on MMSE, 21 percent, and on ADL, 23 percent. As with the iADRS, active groups first diverged from placebo at 24 or 36 weeks. Mintun noted that the 23 percent slowing on CDR-SB is no smaller an effect size than has been seen to date in AD trials.
Among individual participants, the pattern of plaque clearance varied, with some getting a large initial drop and others a steady decline. This made no difference to the cognitive benefit, Mintun said.
Given that donanemab completely cleared plaque, the researchers acknowledged that a 32 percent slowing may represent the most it can achieve in people at this stage of AD. “This is probably the ceiling for an amyloid-lowering drug,” Skovronsky said. To do more for patients, researchers likely will have to treat earlier in a prevention paradigm, or combine anti-amyloid treatment with an anti-tau drug, he suggested.

Less Tangles, More Cognition. Trial participants with the lowest tangle burden (left) reaped the biggest benefit, while those with the most tau pathology (right) had none. The findings may help refine selection for future trials. [Courtesy of Eli Lilly.]
When Plaques Vanish, Tangle Formation Slows
About that tau … unlike the loose association amyloid has with cognition, tau tangles are closely linked to cognitive decline. Did donanemab affect them? On a measure of global tau PET, the answer was no. Tau tracer uptake climbed in both groups throughout the study, with the active treatment group gaining only 10 percent less than the placebo group, a nonsignificant difference.
When the researchers looked at regional tracer uptake, they saw something different. Tangle accumulation slowed by 59 percent in the frontal lobe, by 45 percent in the parietal lobe, and 32 percent in the lateral temporal lobe. These differences were statistically significant, the first two at p=0.002. In mesial temporal lobe, the placebo group had no change in tangles, but the donanemab group saw a slight but significant drop. The groups were no different in the occipital lobe.
Plaque clearance was linked to the slowing of tau pathology, Mintun noted. Participants who reached amyloid-negative status during the trial had more slowing on tau PET than those who didn’t. It is unclear mechanistically how plaques affect tangles. Mintun suggested that some toxic aspect of amyloid may be responsible for accelerating tauopathy, such that removing it puts on the brakes. A recent study implicated microglial inflammation as the culprit linking the two pathologies (Nov 2019 news).
Regional tangles, particularly in the frontal cortex, also correlated with the cognitive outcome, with a higher baseline frontal tau signal predicting faster decline on the iADRS. “We’ve provided data to validate regional tau spread as an important surrogate for disease progression and drug effect,” Skovronsky said.
What about the idea that baseline tangle load influences donanemab’s effect? To study this, the researchers stratified the active group into terciles based on their baseline tau PET. The lowest tercile, below 1.14 SUVR, drove most of the cognitive benefit from donanemab; this group’s decline slowed by almost half. The intermediate tercile showed little treatment benefit, and the highest, above 1.27 SUVR, none (see image above). These subgroups were too small for statistical significance, and the analysis is exploratory, Mintun said.
Nonetheless, Lilly researchers believe the data may help explain why this trial succeeded. “Excluding patients with high tau could be a key factor in the efficacy of donanemab. We believe it’s important that all future Alzheimer’s trials and therapies be based on the pathological stage of the patient, as is done in oncology,” Mintun said.
Others wondered whether the tau range should be narrowed further for Phase 3, since people with an SUVR between 1.27 and 1.46 did not benefit. Skovronsky said Lilly will keep the range the same for Phase 3. The idea is to replicate the Phase 2 findings, and Phase 3 will have more power to detect treatment effects.
Gil Rabinovici of UCSF was intrigued by these data. “This suggests that the primary role of amyloid-lowering therapies may be in patients in whom tau is not yet widespread, most of whom will be in the preclinical or very earliest clinical stage. Progress in blood-based biomarkers should greatly facilitate the detection of earliest-stage AD in an accessible, equitable and cost-effective manner,” he wrote (full comment below).
ARIA Still An Issue
Safety data in the Trailblazer trial was similar to previous donanemab studies and to other antibodies in this class. A quarter of the active group, 35 people, developed the brain edema known as ARIA-E. In eight people, about 6 percent of those on donanemab, it was symptomatic. People taking the antibody also developed more superficial siderosis, iron deposits that form on subpial surfaces due to small brain bleeds, than controls, at 14 percent versus 3. They had more ARIA-H, or microhemorrhages, at 8 percent versus 3, and more nausea, 11 percent versus 3. Donanemab administration caused infusion reactions in 10 people, though most were mild or moderate, did not require intervention, and did not reoccur. There were no differences in serious adverse events or deaths between groups.
Erik Musiek of Washington University, St. Louis, noted that the ARIA-E incidence was similar to that in people taking low-dose aducanumab, while the ARIA-H incidence was lower than the 17 percent reported for aducanumab (comment below). Rabinovici believes the overall safety profile of donanemab would be acceptable to most AD patients.
More people on donanemab than placebo, 40 versus nine, discontinued treatment, most due to ARIA-H or superficial siderosis. Most who stopped treatment remained in the study, and their data were included in the final outcome measures. Skovronsky said that those who stopped treatment due to ARIA had already achieved a high degree of plaque clearance, and attained the same drug benefit as those who remained on therapy.
Russell Swerdlow of University of Kansas Medical Center, Kansas City, noted that the occurrence of symptomatic ARIA can confound trial results by inadvertently unblinding participants. Because APOE4 carriers are more likely to develop ARIA and stop treatment, the removal of such fast progressors from the treatment arm also could skew results. “Hopefully the planned Phase 3 studies will implement measures to take into account these potential confounders,” Swerdlow wrote (full comment below).
Lilly researchers contend that ARIA barely affected their Phase 2 results, since an analysis of donanemab subgroups with and without ARIA-E found no difference in their respective rates of decline, and people who stopped treatment were included in the final analysis.
As in the earlier donanemab trial, 90 percent of participants developed anti-drug antibodies. These did not appear to affect treatment efficacy, but Skovronsky acknowledged that it would be better to have a treatment that does not produce them. Lilly is testing such a version of donanemab, dubbed N3pG4, in clinical trials, but Skovronsky said Lilly intends to bring donanemab to market.
Phase 3: Stick With What Worked
Based on the Trailblazer data, Lilly researchers have made several changes to Trailblazer 2, which has been enrolling since last summer. Instead of a Phase 2 trial with 500 participants, it will become a Phase 3 with 1,500. Scientists hope the larger sample will boost the chance of success on secondary outcomes, increase power to see subgroup effects including in the tau tercile groups, and generate a larger safety database, Mintun noted. Trial sites have been enrolling people with both intermediate and high tau scans, but will now limit the primary efficacy analysis to 1,000 participants with intermediate tangle pathology. Data from 500 people with a higher tangle load, above 1.46 SUVR, will help inform future treatment guidelines, Mintun said.
Lilly had previously considered using the CDR-SB as an endpoint for the new trial, but will stick with iADRS instead. In answer to an investor question, Skovronsky said this was discussed with regulators at the Food and Drug Administration. In one change from Phase 2, however, Lilly will analyze efficacy using the Disease Progression Model (DPM), which generates a probability of disease progression based on data from every timepoint across the trial, rather than looking only at the last timepoint, as the standard model does. Mintun said the final timepoint in AD trials is often the noisiest, so he believes a DPM analysis will be more reliable. In the Phase 2 trial, a DPM analysis gave similar results to the standard method, with every endpoint favoring donanemab.
The Global Alzheimer’s Platform will help speed recruitment for Trailblazer 2. GAP co-founder John Dwyer, who leads the Washington, D.C.-based organization, noted that the original Trailblazer was the first trial GAP worked on. His goal is for Trailblazer 2 to enlist 130 sites worldwide, including many in the GAP-North American network. “We expect to have an immediate impact on TRAILBLAZER-ALZ 2, and one of our priorities will be reaching potential participants from diverse communities,” Dwyer wrote to Alzforum.
Skovronsky expects Trailblazer 2 to complete enrollment in the second half of 2021 and read out in the first half of 2023. Lilly will explore its chances for accelerated regulatory approval, but Trailblazer 2 will complete regardless. “Replication will answer important questions for the field, such as confirming subgroups that show no benefit,” Skovronsky said. Still, because the first Trailblazer was designed as a registration trial, he expects a second positive trial could be sufficient for approval.
If donanemab only helps people up to a certain tangle load, how large is the estimated patient group? Skovronsky noted that 4.5 million people have early symptomatic AD in the United States, 5 million in Europe, and 4 million in Japan. If 30 to 45 percent meet the criteria, that would be 1–2 million people in each place.
The FDA is considering a licensing application from Biogen for its anti-amyloid antibody aducanumab (Feb 2021 news). Will the donanemab data influence the agency? Rabinovici, at least, thinks it should not. “While these results are encouraging for the overall drug class, the FDA needs to consider the aducanumab EMERGE and ENGAGE data on their own merits. There are significant differences between aducanumab and donanemab in the antibody-targeted epitopes, study design and patient populations, and one cannot generalize results from one trial to the other,” he wrote.
Others think eventual approval of an anti-amyloid therapeutic is now inevitable. “The donanemab results provide powerful support for the amyloid therapeutic hypothesis; this strategy will bring the first disease-modifying drugs for AD into clinical use,” Paul Aisen of the University of Southern California, San Diego, wrote to Alzforum (full comment below).—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- In Phase 2, Donanemab Curbs Cognitive Decline in Early Alzheimer’s
- Chicago: Interest in PyroGluAβ Flares Up in Academia
- San Diego: Pilin’ on the Pyro, Aβ Going Rogue
- Could Antibodies Against Pyroglutamate Safely Break Down Plaques?
- Four Immunotherapies Now Banish Amyloid From the Brain
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
- Teasing Out the Brain Features Behind Cognitive Reserve
- Microglia Inflammasome Stokes Tau Phosphorylation, Tangles
- Aducanumab Decision Delayed for Three Months
Paper Citations
- Wessels AM, Siemers ER, Yu P, Andersen SW, Holdridge KC, Sims JR, Sundell K, Stern Y, Rentz DM, Dubois B, Jones RW, Cummings J, Aisen PS. A Combined Measure of Cognition and Function for Clinical Trials: The Integrated Alzheimer's Disease Rating Scale (iADRS). J Prev Alzheimers Dis. 2015 Dec 1;2(4):227-241. PubMed.
Other Citations
External Citations
Further Reading
News
- Anti-Amyloid Therapies Combine Forces to Knock Out Plaques
- New Alzheimer’s Drug Shows Safety, Hints of Efficacy in Phase 2
- Topline Results: 18 Months of BAN2401 Might Work
- BAN2401 Removes Brain Amyloid, Possibly Slows Cognitive Decline
- Four Immunotherapies Now Banish Amyloid From the Brain
- Second Look at BAN2401 Data Still Positive, Despite Snafu
- Exposure, Exposure, Exposure? At CTAD, Aducanumab Scientists Make a Case
- FDA Advisory Committee Throws Cold Water on Aducanumab Filing
- Aducanumab Still Needs to Prove Itself, Researchers Say
Primary Papers
- Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCSF
I am very encouraged. This trial met its prespecified primary endpoint, which I believe is a first for an anti-amyloid drug in our field. In addition, the growth of tau, measured by tau PET in the frontal lobes, was slowed; this provides evidence of disease modification.
Finally, their protocol did not require monoclonal antibody infusions throughout the study, but dosed participants for six to 12 months and then stopped for almost all participants once their amyloid load was brought down to “normal” levels. In my view, this study, together with data from other trials, strongly confirms the “amyloid hypothesis” and demonstrates that treatments aimed at amyloid can slow cognitive decline and modify the progression of AD.
UCSF
Saturday was a very exciting day! The donanemab Phase 2 study offers some of the most compelling evidence to date that amyloid reduction can slow clinical decline, albeit modestly, in early stages of AD. While we have seen a suggestion of similar results before with aducanumab and lecanemab, these trials had known limitations, whereas the donanemab data are more straightforward to interpret. The safety profile is similar to what has been reported previously with potent anti-Aβ antibodies in terms of ARIA-E and ARIA-H, and in my opinion would be considered acceptable by most patients.
In addition to the encouraging results, there are two unique features of this trial that move the field forward. First is the decision by design to reduce dose or switch to placebo once patients are in the amyloid-negative range on PET. This approach has tremendous appeal when considering infusion therapies, and I hope it proves effective.
The second is the selection of patients with intermediate tau on PET. This, too, has strong biological face validity, though one can imagine the significant issues it would raise in clinical implementation. It is interesting that the overall clinical benefit was driven by the patients in the lowest tau PET tertile. This suggests that the primary role of amyloid-lowering therapies may be in patients in whom tau is not yet widespread, most of whom will be in the preclinical or very earliest clinical stage. Progress in blood-based biomarkers should greatly facilitate the detection of earliest-stage AD in an accessible, equitable, and cost-effective manner.
While these results are encouraging for the overall drug class, the FDA needs to consider the aducanumab EMERGE and ENGAGE data on their own merits. There are significant differences between aducanumab and donanemab in the antibody-targeted epitopes, study design and patient populations, and one cannot generalize results from one trial to the other.
Mayo Clinic College of Medicine
This study used a straightforward trial design with a clear outcome, which met the prespecified outcomes. The rate of amyloid-related imaging abnormalities was not trivial but the consequences were manageable. The effect size of reduction of decline was small, and it remains to be seen if a) the result can be replicated, b) whether the result is durable, c) whether the broader community views the benefit as worthwhile.
There is no question that the drug hits its target: The degree of amyloid-lowering is impressive, and the fact that patients could be withdrawn from the treatment is a remarkable prospect for broader use. I am ordinarily skeptical of secondary analyses, but in this case, the regional reductions in tau at least suggest that there was some downstream effect on tau.
The donanemab story is the most encouraging news on the amyloid front, ever, but whether the effect size is clinically meaningful is questionable. Furthermore, given the dramatic effects on amyloid by donanemab, it seems unrealistic to expect that there is some further way to enhance the benefit of an amyloid-lowering drug.
By way of disclosure, I am a site investigator for the A4 study, which is funded largely by Lilly.
University of Kansas
In any therapeutic trial, of course it is much nicer to detect a potential signal of efficacy than not to detect such a signal. The more limited that signal is, though, the more cautious we have to be in interpreting its basis.
One possibility here is that donanemab truly slowed cognitive decline in Alzheimer’s disease. However, as is the case with other trials of amyloid-β-targeted antibodies, individuals with APOE4 alleles are much, much more likely to develop ARIA than those without APOE4 alleles. This could lead to a selective elimination of APOE4 carriers from the active treatment arm, which is relevant because AD patients who are APOE4 carriers may decline more rapidly than AD patients who are not APOE4 carriers (see Figure 1C in Petersen et al., 2005).
ARIA of APOE4 carriers in the treatment arm could also lead to their unblinding, which the authors acknowledge. Table 2 from the Mintun et al. report shows 33 out of 93 APOE4 carriers experienced ARIA-E. Only one out of 90 APOE4 carriers in the control group experienced ARIA-E. Hopefully the planned Phase 3 studies will implement measures to take into account these potential confounders.
Regardless of the efficacy question, donanemab certainly did do an impressive job of reducing PET-detectable β-amyloid.
References:
Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ, Alzheimer's Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379-88. Epub 2005 Apr 13 PubMed.
USC Alzheimer’s Therapeutic Research Institute
The Phase 2 donanemab results are exciting and important. This innovative trial of a highly potent amyloid-normalizing antibody met its primary cognitive/functional endpoint. As with other such antibodies, a significant number of participants developed ARIA, but this was manageable.
It is great news for the field that donanemab has already advanced to a Phase 3 trial. The donanemab results provide powerful support to the amyloid therapeutic hypothesis; this strategy will bring the first disease-modifying drugs for AD into clinical use.
Washington University School of Medicine
The Phase 2 data on donanemab is encouraging and raises expectations for Phase 3 trials. The effect size on the primary endpoint (iADRS score) is considerable and very promising. However, secondary endpoints such as CDR-SB, ADAS-Cog13, and MMSE were less impressive. There was an excellent reduction in amyloid PET signal, though tau PET did not change, and no CSF biomarkers were included.
It is difficult to directly compare TRAILBLAZER (donanemab) to ENGAGE/EMERGE (aducanumab) due to the smaller sample size of TRAILBLAZER and the different primary endpoint, as ENGAGE/EMERGE topline results did not, to my knowledge, include iADRS. EMERGE did show a significant effect on CDR-SB, though the magnitude of effect appears similar to that seen in TRAILBLAZER , though this was not significant in TRAILBLAZER. ENGAGE/EMERGE did show decreases in CSF p-tau markers and tau PET from a small subset of patients, an effect not seen in TRAILBLAZER.
Safety concerns related to ARIA seem to be similar to aducanumab. During the double-blinded period, the proportion of treated patients with ARIA-E with donanemab (26.7 percent) was similar to low-dose aducanumab (25.7 percent) and less than high-dose aducanumab (34 percent), as reported for EMERGE (similar in ENGAGE). ARIA-H was less common with donanemab (8.4 percent) than with low-dose (16.2 percent) or high-dose (18.6 percent) aducanumab.
The current Phase 2 data for donanemab is exciting. The efficacy appears similar to or perhaps greater than aducanumab, though the primary endpoints were different. The safety profile is similar to aducanumab. A potential advantage of donanemab is that antibody dosing may be intermittent, with BACE inhibitor treatment in the interim, which may reduce burden on patients and cost.
Donanemab also engages a different, plaque-related target. Thus, larger studies are certainly warranted and will complement ongoing studies with other drugs, including aducanumab.
Barrow Neurological Institute
I am impressed with the donanemab data for several reasons. The design is informed based on previous studies. The titration up is appropriate. The selection of intermediate tau load is novel.
The pyro-glu epitope is downstream from oligomeric species, suggesting more than one form of amyloid might serve as a target
The study met its prespecified endpoint on the iADRS, a composite measure that is not confounded by difficult-to-interpret analysis, e.g., Bayesian for lecanemab, and futility for aducanumab.
The titration down after amyloid PET is negative is a forward-thinking approach and will be the trend in the future. I look at it as induction therapy. Also, all secondary endpoints trend in the same direction.
This is now the third or fourth mAB to show directional concordance with amyloid removal and clinical signal.
Mayo Clinic
I think the donanemab data are interesting and possibly encouraging. The key features are:
The clinical endpoint was important, and the implication of the discontinued dosing once the participant’s amyloid level normalizes may suggest that, if replicated, we will not need to treat patients continuously. We may be able to lower their amyloid levels, monitor them and, if the levels rise, re-dose. This would be akin to giving a booster immunization.
All of this gives me cautious optimism about donanemab’s potential. I was not involved in the study and do not consult for Lilly.
Lund University
There are several very interesting aspects of the design of the TRAILBLAZER-ALZ study. The approach to reduce, or even stop, treatment when amyloid PET imaging shows that the drug target has been sufficiently diminished (or maybe even eliminated) will substantially facilitate implementation of this treatment in clinical practice.
Even more importantly, this approach really opens up treatment of AD during the preclinical phase of the disease. In this phase, continuous treatment with immunotherapy for five to 20 years would not be an option for the huge majority of affected individuals. However, treatment of individuals with preclinical AD with donanemab for six to 12 months, until their amyloid PET has normalized, would probably be tolerable for most and might also be cost-effective.
Following treatment, plasma levels of p-tau, and/or Aβ42/Aβ40, could be analyzed every three to six months, and when the plasma markers start to revert back to abnormal levels, a new amyloid PET could be done, triggering temporary treatment with donamemab if the amyloid PET scan reveals recurrent accumulation of Aβ fibrils.
It will be very interesting to see the plasma biomarker data from the TRAILBLAZER-ALZ and the TRAILBLAZER-EXT studies to better understand how plasma p-tau and Aβ42/Aβ40 change in response to treatment, and how they relate to changes in amyloid PET in this setting.
A potential worry is that the effects of the treatment on cognition and ADL function resembles those achieved by symptomatic treatment with cholinesterase inhibitors. The current results imply that removal of Aβ fibrils might improve neuronal function, but the rate of cognitive decline after six to eight months of treatment seems to be the same as in the placebo group. If the same results are obtained in the follow-up study (TRAILBLAZER-ALZ 2), then it will be important to better understand the reasons behind this finding.
It could be that the Aβ fibrils themselves, or the oligomers formed on the surface of the Aβ fibrils, have direct toxic effects that are mitigated by the treatment, resulting in a relatively improved cognitive function during the six- to eight-month period when most Aβ fibrils are removed from the brain. However, after that six- to eight-month period the cognitive decline seems to continue at a rate similar to the placebo group, which might imply that Aβ fibril-independent toxic mechanisms are at play at this late stage of the disease.
It is possible, but not yet proven, that upstream events in AD, like Aβ pathology, might trigger downstream events where the latter eventually becomes independent from the initiating event. If this is true, then treatment during the preclinical phase of the disease should be clearly more effective. The preliminary results of the donenemab trial, indicating that individuals with a higher burden of tau pathology, i.e., those at a later stage of the disease, did not respond as well to treatment, could support this theory.
In conclusion, I think the results of the TRAILBLAZER-ALZ are very promising. I am really looking forward to a cleverly designed trial in preclinical AD with this drug, e.g., including individuals with abnormal amyloid PET scans, and potentially also increased P-tau levels, but as yet no or limited neocortical tau PET signal.
Florey Institute of Neuroscience and Mental Health
I was sadly disappointed by the study data. It is curious that there has been little discussion about the acceleration to brain-volume loss (whole brain and ventricular enlargement) in patients treated with donanemab. To preempt the tortured rationalization that this may reflect reduction of plaque load, the total Aβ load in the human brain with AD is ~6.5mg (Roberts et al., 2017), the difference in whole brain volume between placebo and donanemab was ~5ml (Fig 3C, Mintun et al., 2021). It is inconceivable that a reduction in Aβ could account for this difference.*
A better explanation is that donanemab induced brain damage, but not to the hippocampus, where no difference was detected. Several other anti-amyloid drugs have also reported accelerated brain volume loss, which could be a worrying class effect.
These anatomical findings are a caveat to the cognitive/functional results, which, on a generous read, are not strong. Donanemab seemed to merely delay decline by ~six months. Disease progression seems to ensue without altered trajectory. While we welcome any positive impact to cognition, the effect appears modest, and our enthusiasm must be tempered by a marginal p value for iADRS and that all other tests were nonsignificant.
As others have noted, this was a well-performed study, with reasonable N, and stringent selection criteria. If donanemab had a clinically meaningful effect, we would expect to see this reflected in the statistical analysis of the other clinical measures. If we do not observe significance in a highly selected cohort, the chances are low of this drug meaningfully impacting the heterogenous clinical population in our societies.
* If we assume that all Aβ in the brain is insoluble, therefore taking up volume, and that donanemab removed all 6.5 mg of Aβ protein in the brain, and that the specific density of protein is 1.35g/cm3, then we can calculate that, at most, Aβ would occupy 0.0048 cm3 = 0.0048 mL. This is 1,000 times less than the change in brain volume reported.
References:
Roberts BR, Lind M, Wagen AZ, Rembach A, Frugier T, Li QX, Ryan TM, McLean CA, Doecke JD, Rowe CC, Villemagne VL, Masters CL. Biochemically-defined pools of amyloid-β in sporadic Alzheimer's disease: correlation with amyloid PET. Brain. 2017 May 1;140(5):1486-1498. PubMed.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704. Epub 2021 Mar 13 PubMed.
Henan Academy of Innovations in Medical Science
How can anyone claim that this study confirms the amyloid hypothesis? While donanemab is able to clear the brains of patients of amyloid very effectively, the impact on cognition is only marginal, and perhaps of little relevance in the daily life of AD patients. Surely, if the amyloid theory was right, disease progression should have been halted for good.
Putting ideology aside, the outcome of this trial proves that amyloid plays virtually no role in disease progression of AD. It is time to move on and to focus on other parameters that appear to be of importance in AD, such as chronic inflammation and loss of energy utilization in neurons.
Paris Brain Institute and Neurology department, AP-HP
The donanemab data is both encouraging and a little bit disappointing.
Encouraging because donanemab does the job of amyloid plaque clearance as never before and shows cognitive/functional benefits, albeit modest and with arguable clinical significance. Disappointing because it seems difficult to do better than that on the anti-amyloid front, and the clinical effects are not up to our collective expectations.
So what now? I would be thrilled to see a long-term follow-up study with donanemab. The A4 study that was actively debated when it started now seems like the perfect test of this therapeutic venue but, unfortunately, solanezumab does not seem to be the drug we would choose today.
More and more I wonder about the ability any drug can have to show meaningful efficacy during a three-year follow-up period that is dictated more by financial reasons than by scientific or medical ones. Why not include in these trials at least a subgroup of participants who would agree to undergo a loose, digitally assisted follow-up for up to 10 years, maybe through academic/industrial partnership as in A4? The medical community would have much more confidence in a drug that is able to show a long-term positive impact for AD patients.
University of Southampton, Faculty of Medicine
Université de Paris Cognitive Neurology Center, GHU APHP Nord Lariboisiere Fernand-Widal Hospital
University of Paris Diderot
University of Southampton
Aβ immunotherapy has been considered as a treatment for Alzheimer’s disease for some years; however, the lack of clear cognitive efficiency associated with the presence of side effects in some patients has tempered enthusiasm. Nevertheless, the recent publication of a clinical trial of passive Aβ immunotherapy targeting a specific form of post-translationally modified Aβ has confirmed that clearing Aβ seems to slow the cognitive decline in patients treated at a relatively early stage of the disease.
Two additional observations were highlighted in this publication, consistent with the postmortem studies conducted on the unique cohort of 22 Alzheimer’s patients actively immunized against Aβ42 (AN1792, Elan Pharmaceuticals). Firstly, the treatment not only targets Aβ pathology but also improves tau pathology. Changes in tau pathology were previously reported in immunized patients with resolution of plaque-associated dystrophic neurites and reduction in phosphorylated tau in neuronal processes (Boche et al., 2010), associated with a reduction in the tau-phosphorylating enzyme GSK3β (Amin et al., 2015). Changes were still present 14 years following Aβ immunization with a focal reduction in tangles in the regions free of Aβ plaques (Nicoll et al., 2019).
The second observation relates to the acceleration of brain volume loss in the treated patients concordant with the brain atrophy described on in vivo MRI (Fox et al., 2005), and postmortem (Paquet et al., 2015) after active Aβ immunotherapy. Neuronal loss observed as increased interneuronal distance, reduced neuronal density, and increased cerebral cortical neuropil degeneration were enhanced in the brains of immunized Alzheimer’s patients relative to non-immunized patients (Paquet et al., 2015). However, these studies also reported improved health of the residual neurons with better neuritic curvature and less pro-apoptotic neurons in the immunized brains (Paquet et al., 2015; Serrano-Pozo et al., 2010; Paquet et al., 2017).
Clinically, in the AN1792-treated AD patients, no association between neuronal loss and cognitive decline was detected, while a positive correlation between the number of neurons and the age at death was reported (Paquet et al., 2015). Therefore, the brain volume loss could, at least partially, be explained by a donanemab-induced reduction of injured neurons with, as in AN1792, a predicted decreased expression of apoptotic proteins in the brains of treated patients.
To conclude, Aβ immunotherapy has likely beneficial effects, clearing the brain of Aβ plaques, reducing Aβ-driven tau accumulation and possibly removing degenerate neurons, but for these effects to impact on cognition, the treatment should be applied at the earliest stages in order to protect the neurons. All the effects detected in imaging the brains of patients involved in clinical trials have been detailed in postmortem studies, and thus a better knowledge and understanding of these neuro-immunopathological changes should be taken in account by the clinical studies (Boche and Nicoll, 2020).
References:
Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, Nicoll JA. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer's disease. Acta Neuropathol. 2010 Jul;120(1):13-20. PubMed.
Amin J, Paquet C, Baker A, Asuni AA, Love S, Holmes C, Hugon J, Nicoll JA, Boche D. Effect of amyloid-β (Aβ) immunization on hyperphosphorylated tau: a potential role for glycogen synthase kinase (GSK)-3β. Neuropathol Appl Neurobiol. 2015 Jun;41(4):445-57. PubMed.
Nicoll JA, Buckland GR, Harrison CH, Page A, Harris S, Love S, Neal JW, Holmes C, Boche D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer's disease. Brain. 2019 Jul 1;142(7):2113-2126. PubMed.
Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005 May 10;64(9):1563-72. PubMed.
Paquet C, Amin J, Mouton-Liger F, Nasser M, Love S, Gray F, Pickering RM, Nicoll JA, Holmes C, Hugon J, Boche D. Effect of active Aβ immunotherapy on neurons in human Alzheimer's disease. J Pathol. 2015 Apr;235(5):721-30. Epub 2015 Jan 7 PubMed.
Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP, Hyman BT. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010 May;133(Pt 5):1312-27. PubMed.
Paquet C, Nicoll JA, Love S, Mouton-Liger F, Holmes C, Hugon J, Boche D. Downregulated apoptosis and autophagy after anti-Aβ immunotherapy in Alzheimer's disease. Brain Pathol. 2017 Oct 13; PubMed.
Boche D, Nicoll JA. Invited Review - Understanding cause and effect in Alzheimer's pathophysiology: Implications for clinical trials. Neuropathol Appl Neurobiol. 2020 Dec;46(7):623-640. Epub 2020 Jul 25 PubMed.
Make a Comment
To make a comment you must login or register.