New at Tau2020: PET Detects First Traces of Tangles in Rhinal Cortex
Quick Links
Tau2020, the first of what may become a regular “pan-tau” conference (see Part 1 of this series), got off to a brisk start February 12–13 in Washington, D.C. Researchers who focus on primary tauopathies, such as progressive supranuclear palsy, rubbed shoulders with others who study secondary tauopathies, such as Alzheimer’s disease (AD).
- In Alzheimer’s, PET first detects tau deposits around the rhinal sulcus.
- It then spreads to the inferior temporal lobe and precuneus.
- The pattern may provide a staging scheme for tau PET.
As with any tau conference these days, PET imaging featured prominently. The session showed how unequal the field of tauopathy imaging has been of late. AD researchers have reasonably well-characterized ligands that bind AD neurofibrillary tangles with respectable specificity and sensitivity, and they use them in both natural history studies and clinical trials. Alas, those ligands work poorly in the PSPs and CBDs of the world. In D.C., scientists described newer tracers, such as APN-1607 and CBD2115. How well these will perform remains to be seen (see Part 4 of this series).
AD researchers are using PET to figure out how the disease causes cognitive decline. In D.C., Keith Johnson of Massachusetts General Hospital, Boston, reviewed data from the Harvard Aging Brain Study (HABS) that correlated longitudinal change in amyloid plaques, neurofibrillary tangles, and cognition. This data fits with the field’s majority view of a sequential model of progression, whereby baseline amyloid load drives both plaque and tangle accumulation, and the latter drives cognitive decline (Jun 2019 news; Feb 2020 conference news).
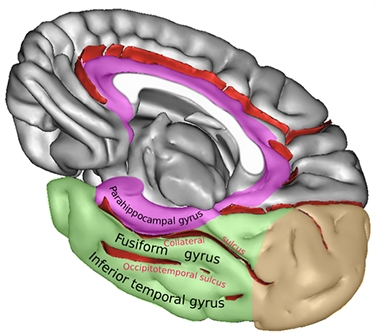
Rhinal Sulcus. The rhinal sulcus comprises the anterior section of the collateral sulcus. It separates the entorhinal part of the parahippocampal gyrus from the fusiform gyrus. [Courtesy of Sebastian23, Creative Commons license.]
But where in the brain does tau accumulation start? In D.C., Johnson suggested that, at least as visible by PET, it may be in a speck of brain he calls the “rhinal cortex.” The rhinal cortex isn’t a histopathologically defined region, said Johnson. Rather, it is a small area surrounding the temporal lobe’s rhinal sulcus and is delineated purely based on the resolution of PET imaging. The rhinal sulcus, which some consider an extension of the collateral sulcus, is the fissure separating the entorhinal cortex from the temporal pole.
Using flortaucipir PET imaging in a cross-sectional study of healthy controls and older people from the A4/LEARN clinical trial, Justin Sanchez in Johnson’s lab found that tangles begin to accumulate in this rhinal cortex in people who have only a low amyloid burden. Once amyloid starts to build, then the spread of tangles takes off in the temporal lobe. As the disease progresses, tangles invade the precuneus.
Based on this data—and on Gaussian mixture modelling, which finds sub-distributions among larger probabilistic outcomes—Sanchez then identified four tau PET staging thresholds. Called Tp0 to Tp3, they reflect no specific flortaucipir uptake, binding in the rhinal cortex, binding in rhinal cortex plus inferior temporal lobe, and binding in rhinal cortex, inferior temporal lobe, and precuneus, respectively.
This staging trajectory replicated in 104 volunteers in HABS, said Johnson. The tau stages correlated with Aβ burden, he added, noting that the scheme could become a tool to stage early tau pathology in living people.
Will this staging scheme distinguish age-related from disease-related tau accumulation? Johnson is trying to address this question. Alas, it is confounded not only by pathology, but also by resilience, genetic, and other factors. “We may be able to transform the data to have some diagnostic value, but that will be hard to do,” he said.
Is the rhinal cortex ground zero for tau deposition? Maybe not quite. Histopathology suggests that it begins even earlier, even deeper in the brain, i.e., in the locus coeruleus (Braak et al., 2011). Alas, this has been difficult to detect with current tau tracers. Therefore, Heidi Jacobs in Johnson’s lab decided to probe the locus coeruleus by measuring how it held up as tau pathology progressed.
She measured its size with MRI and correlated that data with flortaucipir binding in the cortex. In D.C., Johnson reported that the integrity of the locus coeruleus correlated inversely with tangle burden in the entorhinal and medial prefrontal cortex. This supports the idea that a flagging locus coeruleus may be a harbinger of AD neuropathology.—Tom Fagan
References
News Citations
- Tau2020: Meeting for Tauopathies Debuts Genetic Variants
- Primary Tauopathies Get New PET Ligands
- Serial PET Nails It: Preclinical AD Means Amyloid, Tau, then Cognitive Decline
- How Much Amyloid Will Kick Off Tangles, and Decline?
Paper Citations
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-9. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.