Shape of α-Synuclein Aggregates Influences Pathology
Quick Links
Growing evidence suggests that structurally different aggregates of misfolded protein may underlie some of the bewildering heterogeneity with which neurodegenerative diseases express themselves in patients. In the June 10 Nature online, researchers led by Ronald Melki at the French National Center for Scientific Research, Gif–sur–Yvette, France, and Veerle Baekelandt at KU Leuven, Belgium, make a case that this is true in synucleinopathies. They report that injecting different types of α-synuclein aggregate into rat brains resulted in distinct pathologic consequences. Flat, ribbon-like aggregates gave rise to Lewy bodies and glial inclusions similar to those seen in multiple-system atrophy, while cylindrical fibrils triggered the degeneration of dopaminergic neurons and motor problems like those that bedevil Parkinson’s patients. “The structural differences [of aggregates] define their propensity to target different cells and circuits within our brains,” Melki wrote to Alzforum.
“These results suggest that different synucleinopathies are defined by different strains of α-synuclein aggregate,” wrote Seung-Jae Lee at Seoul National University College of Medicine and Eliezer Masliah at the University of California, San Diego, in an accompanying News & Views commentary.
Several studies support the existence of distinct strains of misfolded protein, which then propagate themselves through the brain by seeding (see Apr 2015 conference news). So far, little is known about how such strains might affect pathology and behavior. Researchers at the University of Minnesota, Minneapolis, recently reported that two different structural classes of Aβ oligomer produced distinct effects in vivo, and different strains of both Aβ and tau have been found in human tissue (see Jun 2015 news; Sep 2013 news; May 2014 news). For α-synuclein, researchers reported that certain aggregate structures can seed tau tangles, while others drive α-synuclein deposits (see Jul 2013 news). Melki had previously distinguished between two classes of α-synuclein aggregate, ribbons and fibrils. In cell cultures, fibrils were more toxic and seeded longer-lasting aggregates than ribbons (see Bousset et al., 2013).
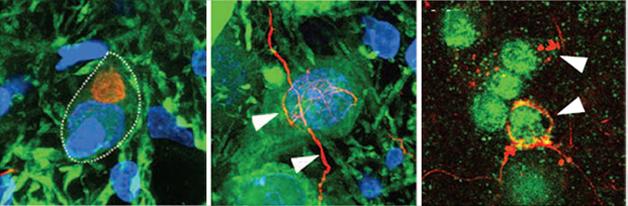
Ribbons Seed Pathology. In rats injected with α-synuclein ribbons, characteristic α-synuclein deposits (red) form in dopaminergic neurons (left panel), in dopaminergic axons (middle), and in oligodendrocytes (right). [Courtesy of Peelaerts et al., Nature.]
The authors wondered if the two forms might also cause different pathology in the brain. First author Wouter Peelaerts generated oligomers, ribbons, and fibrils from synthetic α-synuclein in vitro using different incubation conditions. The authors then injected 10 μg of each preparation into the substantia nigra of wild-type rats. Over the course of a week, all three assemblies were taken up by dopaminergic neurons, transported anterogradely down axons, and internalized by other neurons. Oligomers dispersed farther through the brain than ribbons or fibrils did. This may be due to their smaller size, Melki suggested.
Four months later, the effects of each kind of aggregate varied widely. Ribbons spurred the formation of Lewy bodies and Lewy neurites in dopaminergic neurons, but had no other ill effects. Fibrils did not seed Lewy bodies, but were associated with a 30 percent loss of dopaminergic neurons, and about a 25 percent loss of motor control in the rats’ forepaws, even in wild-type rats. Surprisingly, oligomers caused no problems in this study, belying previous reports that these forms precipitated the most cellular stress and death (see Nov 2009 conference news; Mar 2012 news).
Would these effects be more severe in a background of α-synuclein overexpression? The scientists injected each type of aggregate into transgenic rats that overexpress wild-type human α-synuclein, lose dopaminergic neurons, and develop motor impairments with age (see Van der Perren et al., 2015). In these animals, the ribbons mildly exacerbated both neurodegeneration and motor phenotype, demonstrating that they can be toxic. Ribbons also produced more Lewy bodies in the transgenics than in wild-type rats, and seeded glial cytoplasmic inclusions in oligodendrocytes as well. Such inclusions are a mark of multiple-system atrophy.
Fibrils again inflicted the largest hit on neurodegeneration, greatly accelerating this process. Among dopaminergic neurons, about half died, nerve terminals in the striatum disappeared, and the rats lost about 75 percent of control in their forepaws. Fibrils seeded sparse Lewy bodies in the transgenic rats. In the synuclein-overexpression model, unlike wild-type, injected oligomers did have an effect, worsening dopamine neuron loss and motor problems.
Overall, the findings highlight that α-synuclein fibrils are the form most toxic to neurons, Melki wrote to Alzforum. He speculated that the data tie fibrils to degeneration and Parkinson’s disease, while ribbons may give rise to multiple-system atrophy. In future work, Melki will examine whether α-synuclein assemblies from the brains of patients with synucleinopathies follow this pattern. To try to learn how each type of aggregate penetrates cells, he will determine the proteins with which they interact. That information could suggest new therapeutic strategies for limiting disease spread, he suggested.
Besides spreading through the brain, could α-synuclein aggregates cross the blood-brain barrier? After injecting each type into the bloodstream of wild-type rats twice weekly over four months, the authors indeed spotted each in their brains. Fibrils accumulated in cortical neurons, spinal cords, and activated microglia.
Kelvin Luk at the University of Pennsylvania, Philadelphia, found the intravenous injection data particularly intriguing. “It suggests there might be ways other than neuron-to-neuron transmission for α-synuclein to disseminate in vivo,” he told Alzforum. Aβ has also been shown to travel from blood to brain to seed amyloidosis (see Oct 2010 news). Overall, Luk called the experiments comprehensive and fascinating, but he noted that researchers still do not know if ribbons and fibrils reflect actual conformations in human disease. A few studies have injected brain material from Parkinson’s or multiple-system atrophy patients into animals and seen pathology, but have not characterized aggregate structures (see Watts et al., 2013; Recasens et al., 2014).
Ole Isacson of Harvard Medical School’s McLean Hospital, Belmont, Massachusetts, was struck by the finding that α-synuclein overexpression alone was more toxic than aggregate injection in these experiments. Several Parkinson’s models employ a similar overexpression approach to trigger disease (see Ulusoy et al., 2010). “This suggests that intracellular, not secreted, accumulation of α-synuclein is the key cell biological driver of pathology in Parkinson’s disease,” he wrote to Alzforum.—Madolyn Bowman Rogers
References
News Citations
- Protein Propagation Real, but Mechanisms Hazy
- Two Classes of Aβ Oligomers Act Differently in the Brain
- Does Aβ Come In Strains? Glimpse Into Human Brain Suggests Yes
- Like Prions, Tau Strains Are True to Form
- An Extra Strain on the Brain—α-Synuclein Seeds Tau Aggregation
- Chicago: Tau and α-Synuclein Oligomers Follow Aβ Footsteps
- Researchers Pinpoint α-Synuclein Oligomers, Link Them to Cell Stress
- Peripheral Aβ Seeds CAA and Parenchymal Amyloidosis
Paper Citations
- Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. PubMed.
- Van der Perren A, Toelen J, Casteels C, Macchi F, Van Rompuy AS, Sarre S, Casadei N, Nuber S, Himmelreich U, Osorio Garcia MI, Michotte Y, D'Hooge R, Bormans G, Van Laere K, Gijsbers R, Van den Haute C, Debyser Z, Baekelandt V. Longitudinal follow-up and characterization of a robust rat model for Parkinson's disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol Aging. 2015 Mar;36(3):1543-58. Epub 2014 Dec 17 PubMed.
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19555-60. Epub 2013 Nov 11 PubMed.
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014 Mar;75(3):351-62. Epub 2014 Feb 18 PubMed.
- Ulusoy A, Decressac M, Kirik D, Björklund A. Viral vector-mediated overexpression of α-synuclein as a progressive model of Parkinson's disease. Prog Brain Res. 2010;184:89-111. PubMed.
Further Reading
News
- Form and Function: What Makes α-Synuclein Toxic?
- Are Synuclein Seeds Non-Starters?
- Alpha-Synuclein Types Congregate in Presynapse—Which Is the Bad One?
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
- Toxic Synuclein Corrupts Native in Wild-Type Mice
- Synthetic Synuclein Corrupts Native Along Mouse Brain Networks
Primary Papers
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015 Jun 18;522(7556):340-4. Epub 2015 Jun 10 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
German Center for Neurodegenerative Diseases (DZNE)
DZNE / Klinikum rechts der Isar der TU München
Hannover Medical School
α-Synuclein aggregates: Different strains for different pathologies?
Synucleinopathies are progressive neurodegenerative diseases with the presence of protein inclusions in brain cells, where the predominant component is α-synuclein. They comprise, among others, Parkinson’s disease, multisystem atrophy, and pure autonomic failure, which can be distinguished from one another neuropathologically by the localization of α-synuclein inclusions in different shapes, in different cell types, and in different anatomical locations in the brain. Mechanisms underlying the tremendous clinical and neuropathological heterogeneity of synucleinopathies remain unclear.
The discovery that fibrils formed of recombinant α-synuclein could spread to previously healthy neurons and act as seeds in cells to induce the recruitment of soluble endogenous α-synuclein into insoluble pathologic aggregates (Luk et al., 2009) paved the way for numerous subsequent studies that investigated the seeding and spreading capacity of different α-synuclein preparations in different experimental settings (Desplats et al., 2009; Hansen et al., 2011).
In this study published recently in Nature, Peelaerts et al. propose that the differences between distinct synucleinopathies may be caused by different "strains" of α-synuclein (Peelaerts et al., 2015).
The notion of α-synuclein strains has been proposed previously by several groups. They have been conceptualized as conformational variants of α-synuclein with different seeding properties in cellular and organismal contexts (Guo et al., 2015). Furthermore, it has been previously shown that different strains of α-synuclein, namely ultrastructurally defined fibrils and ribbons, can be obtained in vitro by applying different oligomerization conditions. These strains showed differences in size, structure, toxicity, lipid-binding potential, and seeding potential in a cell model (Bousset et al., 2013).
Following up on these findings, Peelaerts et al. investigated whether these strains caused different biological changes and pathologies in rats. The in vivo properties of fluorescently labeled α-synuclein oligomers and two distinct strains, fibrils and ribbons, were compared by inoculation into the rat substantia nigra in the absence or presence of α-synuclein overexpression. In this experimental setting, oligomers and ribbons showed more spreading potential than fibrils, but only ribbons were able to cause large deposits of phosphorylated α-synuclein that shared properties with Lewy bodies and Lewy neurites defining Parkinson’s disease. On the other hand, fibrils had the most pronounced neurotoxic effects and were able to induce neurodegeneration of dopaminergic neurons with a motor deficient phenotype. These findings are consistent with previous studies in mice (Luk et al., 2012; Masuda-Suzukake et al., 2014). More strikingly, the injection of ribbons in a system where α-synuclein is overexpressed led to the appearance of phosphorylated α-synuclein deposits in oligodendroglial cells, whereas fibrils caused depositions only in neurons. Whether these deposits share common properties with the glial cytoplasmic inclusions defining multisystem atrophy is not clear, but this finding supports the hypothesis that different strains acting under different conditions cause different pathologies.
Moreover, the proteinase K resistance of α-synuclein aggregates extracted from brains, which have been inoculated with either fibrils or ribbons, was different. This may imply that the different strains act as seeds and have the ability to imprint endogenous α-synuclein with their respective structures.
This study also shows that systemic administration of α-synuclein strains leads to deposition in the brain, consistent with previous findings where intramuscular and gastric injections of α-synuclein assemblies led to deposition and pathology in the brain (Holmqvist et al., 2012; Sacino et al., 2014). This implies that α-synuclein pathology can be initiated in a peripheral system and then spread to the brain, where it causes neuronal dysfunctions. This clinically relevant issue needs to be further investigated.
These findings shed light on several questions relating to the implications of α-synuclein in different pathologies. The concept of strains seems to be of utmost relevance when it comes to explaining the molecular basis defining distinct synucleinopathies. Fibrils are reported to be the primary culprit for neurodegeneration and behavioral and motor deficits, whereas ribbons seem to be associated with the formation of Lewy bodies and deposits in glial cells, but do not appear to be involved in toxicity. On the other hand, oligomers seem to spread faster and more efficiently than fibrils and ribbons. Oligomers were also able to affect the synaptic transmission to the same extent as fibrils, but surprisingly, contrary to studies that have reported toxicity induced by α-synuclein oligomers (Luth et al., 2014), they did not show any toxic or seeding effects in this model. This could be explained with their faster removal by clearance pathways due to their smaller size, but the toxic potential of oligomers nonetheless needs to be characterized and studied in more detail.
More importantly, this work opens up several questions. Do these strains exist in synucleinopathies in human patients? And how can this issue be addressed? Moreover, in synucleinopathies, both α-synuclein-rich inclusions and cell toxicity are occurring. If it is assumed that ribbons are responsible for depositions and fibrils are responsible for toxicity, then these strains must co-exist in the same diseased system. Do strains collaborate in a fashion that induces a differential pathological process leading to different synucleinopathies? Or is the difference caused by different proportions of strains, which would be induced by different microenvironments of cells? And what role does the genetic background play on the formation of different strains? All these questions need to be investigated in order to have a clearer view of how the heterogeneity of synucleinopathies is generated.
Despite the fact that several groups report that fibrils are the α-synuclein strain responsible for neurodegeneration, an important question needs to be addressed jointly by the field to clarify what defines biologically active fibrils: Different groups utilize different α-synuclein preparations with in vitro oligomerization conducted under different conditions to make fibrils. This raises the question of how to improve the reproducibility of experiments done with α-synuclein strains or seeds. There seems to be a great need to standardize protocols and nomenclature to be able to compare observations across different studies.
References:
Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20051-6. PubMed.
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13010-5. PubMed.
Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011 Feb 1;121(2):715-25. PubMed.
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015 Jun 18;522(7556):340-4. Epub 2015 Jun 10 PubMed.
Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013 Jul 3;154(1):103-17. PubMed.
Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. PubMed.
Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012 Nov 16;338(6109):949-53. PubMed.
Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun. 2014 Aug 6;2:88. PubMed.
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014 Dec;128(6):805-20. Epub 2014 Oct 9 PubMed.
Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10732-7. Epub 2014 Jul 7 PubMed.
Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014 Aug 1;289(31):21490-507. Epub 2014 Jun 18 PubMed.
Make a Comment
To make a comment you must login or register.