In Down's Syndrome, Amyloid Vaccine Opens Door to Trials
Quick Links
Part 1 of two. Click here for Part 2.
Alzheimer’s disease and Down’s syndrome are closely related through their β-amyloidosis pathology. Alas, despite dozens of anti-amyloid clinical trials in AD over the past 20 years, there has been but one in Down's, a more ethically fraught field. This Phase 1 study, sponsored by AC Immune, Lausanne, Switzerland, evaluated the active anti-amyloid vaccine ACI-24 in 16 people with DS. The company presented preliminary results in March, reporting that the vaccine was safe and generated anti-Aβ antibodies. Will this first apparent success break down old barriers across the field, and lead to broad-based therapy development in Down's dementia?
- ACI-24 seems safe, generates immune response in people with DS.
- New formulation of this anti-Aβ vaccine to be tested in Phase 2.
- Cohorts and methodologies for additional trials are taking shape.
“This study shows that intervention trials are possible in people with DS, and that they are willing and able to participate,” William Mobley, University of California, San Diego, told Alzforum. "If even one trial can be done safely and effectively in people with DS, it will get the industry excited about further studies."
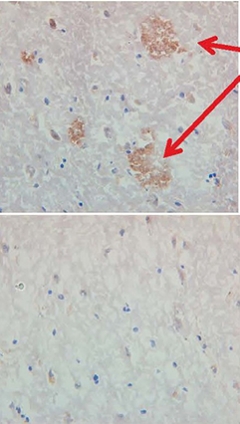
Proof of Concept. Serum from a monkey immunized with ACI-24 binds to amyloid plaques in human brain tissues (top). Serum from a control monkey does not (bottom). [Courtesy of AC Immune, Lausanne, Switzerland.]
Juan Fortea, Hospital of Sant Pau, Barcelona, Spain, agreed, saying companies are showing more interest in funding AD DS trials. “I am convinced this will shortly be a very crowded field,” Fortea wrote to Alzforum.
Down’s syndrome is the most common genetic cause of Alzheimer's dementia. The World Health Organization estimates its incidence as one in every 1,000 live births. Children with DS have three copies of chromosome 21, which hosts the amyloid precursor protein gene. Amyloid plaques start depositing in their brains in their teens and 20s (Lemere et al., 1996; Mori et al., 2002). By age 40, people with Down’s have brain-wide amyloid measurable by PET imaging, meaning they live with a high risk of developing Alzheimer's dementia (Sabbagh et al., 2011; Jennings et al., 2015). Thanks to medical advances, people with DS now live longer than before, and AD has become their main cause of death, with 70 percent having developed dementia before they died (Hithersay et al., 2018). Finding treatments for AD in DS seems more urgent than ever.
However, testing dementia treatments in people with DS is not nearly as straightforward as repeating AD trials. The clinical measures developed to measure efficacy in AD, such as the ADAS-Cog, ADAS-ADL, and even the trusty MMSE, do not work for people with DS because they have grown up with cognitive impairment. Moreover, the extent of their developmental cognitive challenges varies greatly from one person to the next. Cognitive assessments hence needed to be adapted to Down’s.
Likewise, whether the same fluid and imaging markers or protocols as in AD can be used in DS needs to be established. Even obtaining informed consent and assembling an appropriate cohort willing to take part in a trial can be difficult. Still, progress has been made on all these fronts (see Part 2 of this story) and the successful completion of AC Immune's trial may well encourage other sponsors to start.
Anti-Aβ Vaccine Prompts Antibody Response, Company Says
The Phase 1 trial recruited 16 people with Down’s, ages 25 to 45, who did not have dementia, from four clinical centers in the U.S. Participants received either placebo, 300 μg, or 1,000 μg ACI-24 via subcutaneous injection seven times over 12 months, and were followed for a year after their last dose. The company chose an active vaccine rather than a passive immunotherapy because they anticipate that the therapy will have to be given over a long time, and one immunization per year would be more convenient and cost-effective than monthly infusions of antibodies.
Primary outcome measures of this trial were serious adverse events and serum anti-Aβ immunoglobulin G (IgG) titers. For secondary outcomes, the scientists measured CSF anti-Aβ IgG titers; levels of plasma and/or CSF Aβ, total tau, phospho-tau, soluble APP released by α- and β-secretases, and inflammatory cytokines. Also on the list were amyloid-related imaging abnormalities (ARIA), whole brain and hippocampal volume via MRI; cognitive changes as per the Cambridge Neuropsychological Test Automated Battery (CANTAB) and Brief Praxis Test (BPT); changes in behavior via the Vineland Adaptive Behavior Scale (VABS) and Neuropsychiatric Inventory (NPI) scales; and the Clinical Global Impression of Change (CGIC).
Marie Kosco-Vilbois, AC Immune, reported results March 16 at the Global Down Syndrome Forum, an online discussion among scientists and clinicians about the latest research on AD in DS. It was co-sponsored by AC-Immune. Kosco-Vilbois said that the vaccine appeared to be safe. Nobody dropped out or had a serious adverse event. No one developed ARIA, an inflammation of blood vessels in the brain (see company press release). ARIA has become a major safety concern in AD immunotherapy trials. The press release also reports no ARIA or safety concerns in a Phase 2 trial of non-DS people with AD. An interim analysis for that trial is planned for mid-2021.
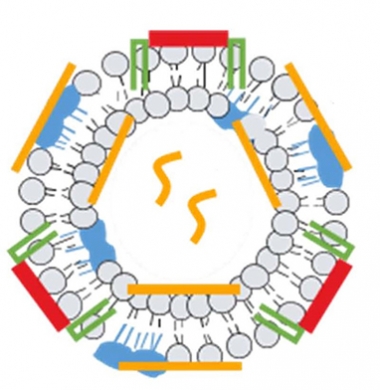
Round Two. In addition to an Aβ fragment (red) and adjuvant (blue), a new version of ACI-24 packs additional immunogenic epitopes (yellow) into a lipid bilayer to stimulate the overall immune response and raise anti-Aβ titers. [Courtesy of AC Immune, Lausanne, Switzerland.]
What about an immune response? According to the company press release, people who received ACI-24 made anti-Aβ antibodies and had higher concentrations of Aβ in their plasma, perhaps an indication that the antibodies were pulling Aβ from the brain. Alas, they presented no CSF data that would support this idea.
A Phase 2 trial of ACI-24 is planned in the U.S. and Europe in 72 people with DS, ages 40 to 50, who have a positive amyloid PET scan but no dementia. At this age, plaques can be detected by PET and dementia symptoms begin to surface. Volunteers will receive vaccine or placebo over 74 weeks, and will be tracked on safety outcomes for 26 weeks after the last dose. The company has not publicly specified dosing or schedule.
Primary measures will reflect safety, such as the number of adverse events, ARIA occurrence, changes in vital signs, and suicidal thoughts or behavior. Secondary outcomes are change in brain amyloid and tau load via PET; serum anti-Aβ IgG titers; change in plasma and/or CSF Aβ, total tau, phospho-tau, and NfL; changes in cognition via CANTAB Paired Associates Learning (PAL) and Cambridge Cognitive Examination–Down Syndrome (CAMCOG-DS); changes in behavior via the Vineland scale VABS; and change in CGIC.
The DS Phase 2 trial is delayed due to COVID. People with DS are at higher risk of COVID-19 infection, complications, and death (Illouz et al., 2021; Hüls et al., 2021).

Immune Response. Serum from a nonhuman primate immunized with a new version of ACI-24 reacts with amyloid plaques in human brain tissue. [Courtesy of AC Immune, Lausanne, Switzerland.]
In the meantime, AC Immune has tweaked the vaccine by adding more adjuvant to boost antibody response. Its researchers tested the new version in four rhesus and four cynomolgus macaques, and report that both primate species produced antibodies that bound to plaques in brain tissue samples taken postmortem from people who had had AD (see image at right).
Are More Anti-Amyloid Therapies Next?
Scientists across the field are contemplating other anti-amyloid therapies in Down’s. In a poster at the International Conference on Alzheimer’s and Parkinson’s Disease (AD/PD) in March, Tomas Odergren, BioArctic, Stockholm, and colleagues showed that people with DS have high levels of soluble Aβ protofibrils in the brain. They suggested that the anti-amyloid antibody BAN2401, which was developed by BioArctic and licensed to Biogen/Eisai, might bind to those fibrils and could be tested in people with DS.
“Several other amyloid-targeting approaches, such as donanemab, may also be promising,” Andre Strydom, King’s College London, wrote to Alzforum. Donanemab was recently shown to slow cognitive decline a bit in people with mild dementia (Mar 2021 conference news).
Other clinical trials are gearing up to test anti-amyloid therapies in adults with DS. Data from longitudinal observational studies in people have taught researchers which fluid, imaging, and cognition biomarkers may work best as AD trial outcomes in DS. This has enabled them to begin recruiting hundreds of participants for DS trial-ready cohorts, aiming to test other anti-amyloid therapies within the next few years (see Part 2). —Chelsea Weidman Burke
References
News Citations
- Gearing Up for Down’s Syndrome Clinical Trials
- Donanemab Confirms: Clearing Plaques Slows Decline—By a Bit
Therapeutics Citations
Paper Citations
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996 Feb;3(1):16-32. PubMed.
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002 Jun;9(2):88-102. PubMed.
- Sabbagh MN, Fleisher A, Chen K, Rogers J, Berk C, Reiman E, Pontecorvo M, Mintun M, Skovronsky D, Jacobson SA, Sue LI, Liebsack C, Charney AS, Cole L, Belden C, Beach TG. Positron emission tomography and neuropathologic estimates of fibrillar amyloid-β in a patient with Down syndrome and Alzheimer disease. Arch Neurol. 2011 Nov;68(11):1461-6. PubMed.
- Jennings D, Seibyl J, Sabbagh M, Lai F, Hopkins W, Bullich S, Gimenez M, Reininger C, Putz B, Stephens A, Catafau AM, Marek K. Age dependence of brain β-amyloid deposition in Down syndrome: An [18F]florbetaben PET study. Neurology. 2015 Feb 3;84(5):500-7. Epub 2015 Jan 7 PubMed.
- Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher EM, Tybulewicz VL, Nizetic D, Strydom A. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. 2018 Nov 19; PubMed.
- Illouz T, Biragyn A, Frenkel-Morgenstern M, Weissberg O, Gorohovski A, Merzon E, Green I, Iulita F, Flores-Aguilar L, Dierssen M, De Toma I, Lifshitz H, Antonarakis SE, Yu E, Herault Y, Potier MC, Botté A, Roper R, Sredni B, Sarid R, London J, Mobley W, Strydom A, Okun E. Specific Susceptibility to COVID-19 in Adults with Down Syndrome. Neuromolecular Med. 2021 Mar 4; PubMed. Correction.
- Hüls A, Costa AC, Dierssen M, Baksh RA, Bargagna S, Baumer NT, Brandão AC, Carfi A, Carmona-Iragui M, Chicoine BA, Ghosh S, Lakhanpaul M, Manso C, Mayer MA, Ortega MD, de Asua DR, Rebillat AS, Russell LA, Sgandurra G, Valentini D, Sherman SL, Strydom A, T21RS COVID-19 Initiative. Medical vulnerability of individuals with Down syndrome to severe COVID-19-data from the Trisomy 21 Research Society and the UK ISARIC4C survey. EClinicalMedicine. 2021 Mar;33:100769. Epub 2021 Feb 22 PubMed.
External Citations
Further Reading
External Links
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.