Do Extended Species of Aβ Poison Synapses, Masquerade As Dimers?
Quick Links
Most studies of Aβ barely look beyond Aβ40 and Aβ42, but researchers have long known that a veritable zoo of APP fragments exist in the human brain. How important are these other species in disease? And how do they change with BACE inhibition? While questions abound, researchers are increasingly debating evidence from groups in Sweden, the United States, and Japan that suggests N-terminally extended (NTE) and N-terminally truncated Aβ peptides deserve further scrutiny. In slice cultures, NTE peptides dampen synaptic plasticity just as Aβ1-42 does. In SDS gels these large species run at the same spot as Aβ1-42 dimers, hinting that some of the toxicity previously attributed to dimers could have been due to these forms instead. For Aβ5-x truncated forms, researchers have not yet studied toxicity but believe they might be particularly prone to aggregate based on their structure. Both NTE and truncated Aβ peptides build up when β-secretase cleavage is suppressed, raising a potential monitoring question for ongoing trials of BACE inhibitors.

Peptide Soup.
Cell cultures produce a rich mix of Aβ species. Some extend N-terminally of the Aβ1-40 start site (orange). Some end at amino acid 15 of the Aβ sequence, others are truncated N- or C-terminally within the Aβ1-42 sequence (blue, green). Many rise after BACE inhibition (arrows on right). [Image courtesy of Erik Portelius.]
Do these peptides play a role in human disease? To date, no evidence of them has surfaced in human brain tissue, though few groups have looked closely. Even so, a study from Erik Portelius, Kaj Blennow, and colleagues at the University of Gothenburg, Sweden, reported the presence of NTE Aβ fragments in cerebrospinal fluid (CSF), and a Japanese group led by Nobel laureate Koichi Tanaka at Shimadzu Corporation, Kyoto, recently reported small NTE Aβs in the blood. The presence of one of these plasma peptides correlated with brain amyloid deposition, Tanaka reported. Complicating matters, all the groups, including researchers led by Dennis Selkoe and Dominic Walsh at Brigham and Women’s Hospital, Boston, have used different systems and detected different menageries of NTE peptides, leaving unclear which ones are most relevant. The Swedish group has also reported finding Aβ5-x in both CSF and plasma from people.
Researchers voiced caution in interpreting these findings, but agreed the issue deserves study. “It is too early to say whether these are important. It would be interesting to characterize these fragments more to see if they are relevant to Alzheimer’s disease,” Gal Bitan at the University of California, Los Angeles, told Alzforum. He was not involved in the research. Selkoe concurred. “We think these NTE-Aβ species are bioactive. They require further identification, isolation, and study in human Alzheimer’s brain tissue to be sure of their actual relevance to pathogenesis,” he wrote to Alzforum.
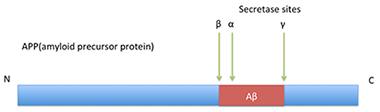
The Basic Idea—But It’s More Complicated Than This.
In classical processing of APP, β-secretase cuts before position 1, α-secretase after position 16 (and possibly also 14 and 15), and γ-secretase snips around position 40, within the transmembrane region.
In canonical Aβ production, β-secretase (BACE1) cuts amyloid precursor protein (APP) just before the aspartic acid 1 site, then γ-secretase cleaves processively within the transmembrane portion of the protein, producing Aβ1-43, Aβ1-42, Aβ1-40, Aβ1-38 and similar fragments. In the alternate, non-amyloidogenic pathway, α-secretase clips APP after position 16 of Aβ, generating harmless species. In recent years, researchers have turned up evidence of a third pathway, β-secretase followed by α-secretase cleavage. It produces Aβ1-16, and may also generate Aβ1-15 and Aβ1-14 (see Apr 2010 news). Aβ1-15 in particular has been associated with improved synaptic plasticity and cognition (see Oct 2014 news). However, the class of Aβ fragments only begins there. Researchers have reported other species of unknown origin, such as the Ab5-x fragments (see Feb 2012 news). Peptides snipped at position 3 and then pyroglutamated have been blamed for seeding amyloid aggregation, and are a target of some antibody therapies (see Mar 2013 conference news).
What’s in That Band: Dimer or Extended Aβ Monomer?
Portelius and colleagues first reported the existence of N-terminally extended fragments in 2009. They immunoprecipitated Aβ from human CSF, then analyzed the peptides by mass spectrometry. They found 11 NTE fragments, all of which ended at position 15, suggesting they might be products of α-secretase cleavage. At the N-terminal end, however, the fragments varied from position -58 to -4. It was unclear what enzyme might produce these, or if they were physiological species or degradation products from a longer peptide (see Portelius et al., 2009; Oct 2009 conference news).
The Swedish researchers followed up by examining Aβ cleavage in the Chinese hamster ovary (CHO) cell line 7PA2 originally developed by Selkoe and colleagues. These widely used cells express human V717F mutant APP, resulting in generation of large amounts of Aβ42. The researchers again found NTE fragments ending at position 15, as well as NTE Aβ peptides that ran all the way to position 40. However, these were just some of a zoo of more than 90 peptide species, which also included classical and N-truncated forms, leaving their significance unclear (see Portelius et al., 2012, and image above).
The discovery of the longer forms raised questions about the interpretation of some previous studies. Earlier work by Selkoe and Walsh had laid the blame for the toxicity of 7PA2 media on Aβ dimers (see Apr 2002 news). Later studies reported toxic dimers in human brain tissue as well (see Nov 2007 conference news; Jun 2008 news). Subsequently, these putative dimers proved difficult to isolate despite years of effort, Walsh told Alzforum. In collaboration with the Swedish researchers, he found that species with the molecular weight of dimers did not behave as expected biochemically. Experiments such as isoelectric point measurement, as well as BACE and cyanogen bromide cleavage, suggested these Aβ species had a primary sequence other than Aβ1-40. The researchers came to suspect that these might instead be extended forms.
Walsh and Selkoe tested this by isolating peptides from the 7PA2 cell media. They found that the gel band that ran at 8.5 kD included only a few genuine Aβ1-40 dimers. Mostly, it consisted of NTE Aβ monomers that extended from about -40 to 40. These peptides inhibited long-term potentiation (LTP) in hippocampal slices, just as purified dimers did. Because the NTE forms made up the majority of the gel band, it is likely that most of the synaptic toxicity in 7PA2-conditioned media comes from this species, rather than dimers, the researchers concluded (see Welzel et al., 2014).
Commentators agreed this finding casts into doubt some previous research conclusions regarding dimers. “This implies the need for a very careful characterization of brain- or cell culture-derived Aβ aggregates prior to their use in different studies,” Iryna Benilova at K.U. Leuven, Belgium, wrote to Alzforum. Bitan noted, “The idea that dimers themselves are particularly important is still questionable.”
Extended Aβs and Human Disease
Are NTE species relevant in people? Tanaka and colleagues suggest they might be. They developed a method to isolate minute quantities of Aβ from human plasma, a technical feat because blood contains about 50-fold less Aβ and about 100-fold more total protein than CSF. To purify Aβ, the researchers precipitated the peptides using magnetic beads coated with two different monoclonal antibodies, then analyzed the sequences with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. This approach detected eight variants of Aβ40 that extended up to nine amino acids N-terminal of the start site, considerably shorter than those reported by the U.S. and Swedish groups, as well as N-truncated forms (see Kaneko et al., 2014).
What do plasma peptides have to do with the brain? That’s unclear, but the Japanese researchers did find that one of the shortest NTE species—Aβ-3-40—correlated so well with cerebral amyloid deposition as measured by PET that it might serve as a surrogate marker. In a study on 40 people who were amyloid-positive and 22 who were amyloid-negative as per PIB PET, a high ratio of Aβ-3-40/Aβ42 picked out the amyloid-positive folks with a sensitivity of 93 percent and specificity of 96 percent (see Kaneko et al., 2014).
The 2009 Swedish paper that examined human CSF also hinted that NTE Aβs might matter in disease. In a pilot study on three people with Alzheimer’s disease and three controls, CSF from patients contained significantly higher concentrations of six of the 11 NTE fragments (mostly the longest ones). The peptides were present at low levels overall, however, with the most abundant one, Aβ-25-15, occurring at 1/5th of the level of Aβ42 in cognitively normal people.
Whether NTE species occur in brain parenchyma remains an open question. Walsh and Selkoe found no evidence of these forms in a recent study of aqueous extracts from human brain (see McDonald et al., 2015). “If the species are there, they are going to be at very low levels,” Walsh told Alzforum, adding that he is continuing to look for them in brain tissue.
BACE Inhibition Stimulates Alternate Cleavages
Another mystery concerns how these NTE species form. Researchers are still chasing the enzymes that cleave upstream of BACE1; candidate proteases include caspases and matrix metalloproteinases. Meanwhile, inhibiting BACE1 seems to pump up this alternate cleavage. Both the Swedish and U.S. groups reported a boost in NTE fragments after treating 7PA2 cultures with BACE inhibitors. Christian Haass at the German Center of Neurodegenerative Diseases, Munich, has presented data on longer NTE species at conferences. He calls them Aη, and reports they end at the β- or α-secretase site. Haass noted that Aη, too, are enhanced by BACE inhibition (see Oct 2014 conference news).
Other studies hint that Aβ5-40 species should be better studied, as well. The Swedish researchers found that these forms rise after BACE inhibition in cell cultures and in CSF from dogs (see Feb 2012 news on Mattsson et al., 2012). These truncated peptides also appear in the CSF of people taking BACE inhibitors (see Portelius et al., 2014). “They are not normally present in CSF,” Blennow told Alzforum. His group has also reported their presence in human plasma (see Pannee et al., 2014).
Several researchers suggested that companies conducting BACE inhibitor trials may want to monitor participants for changes in NTE and truncated species. Walsh told Alzforum his group is developing an immunoassay that will distinguish NTE peptides from Aβ1-x in CSF, making this practical to do. Bitan recommended looking for correlations between levels of NTE peptides and cognitive changes. Vivian Hook at the University of California, San Diego, wrote to Alzforum, “BACE1 inhibitors could be detrimental to AD patients, because an increase in neurotoxic NTE-Aβ may reduce memory function.”
What else might a rise in NTE Aβs do to the brain? No one knows. Walsh has preliminary evidence that these peptides can aggregate to form fibrils and is studying if NTE fibrils influence the aggregation of Aβ1-42 and Aβ1-40. He pointed out that mixtures behave differently than pure preparations, and the human brain contains a complex mixture of different Aβ forms. “The interaction of these different primary sequences could be key to the whole disease process,” he told Alzforum.
Portelius noted that truncated Aβ5-40 species could be aggregation-prone, as well, because they are more hydrophobic than Aβ1-40. Preliminary data from the Swedish group bears this out, Blennow added. A German study reported enhanced aggregation of Aβ4-42, a peptide just one amino acid longer (see Bouter et al., 2013). Truncated forms have been found in amyloid plaques (see Takeda et al., 2004; Portelius et al., 2010).
Whether NTE or truncated Aβs are more synaptotoxic than Aβ1-42 is unknown. Some researchers believe it is the wrong question to ask. “In reality, there are multiple toxic species, all of which may be acting in concert,” Bitan pointed out. Perhaps all of these peptides need to be dealt with to treat AD, he suggested.—Madolyn Bowman Rogers
References
News Citations
- Sweet 16: Novel APP Processing Pathway and a New Biomarker?
- Good Cop, Bad Cop: Can Aβ15 Shield Synapses From Aβ42?
- Not the Usual Suspects: Tracking BACE Inhibition, Axon Role
- Can Dousing PyroGlu-Aβ Treat Alzheimer’s Disease?
- St. Louis: Biomarkers Pre-dementia—Like eFAD, Like LOAD?
- Earliest Amyloid Aggregates Fingered As Culprits, Disrupt Synapse Function in Rats
- San Diego: Oligomers Live Up to Bad Reputation, Part 1
- Paper Alert: Patient Aβ Dimers Impair Plasticity, Memory
- At Birthday Symposium, Massachusetts ADRC Looks to Future With New Data
Mutations Citations
Paper Citations
- Portelius E, Brinkmalm G, Tran AJ, Zetterberg H, Westman-Brinkmalm A, Blennow K. Identification of novel APP/Abeta isoforms in human cerebrospinal fluid. Neurodegener Dis. 2009;6(3):87-94. PubMed.
- Portelius E, Olsson M, Brinkmalm G, Rüetschi U, Mattsson N, Andreasson U, Gobom J, Brinkmalm A, Hölttä M, Blennow K, Zetterberg H. Mass Spectrometric Characterization of Amyloid-β Species in the 7PA2 Cell Model of Alzheimer's Disease. J Alzheimers Dis. 2012 Aug 10; PubMed.
- Welzel AT, Maggio JE, Shankar GM, Walker DE, Ostaszewski BL, Li S, Klyubin I, Rowan MJ, Seubert P, Walsh DM, Selkoe DJ. Secreted amyloid β-proteins in a cell culture model include N-terminally extended peptides that impair synaptic plasticity. Biochemistry. 2014 Jun 24;53(24):3908-21. PubMed.
- Kaneko N, Yamamoto R, Sato TA, Tanaka K. Identification and quantification of amyloid beta-related peptides in human plasma using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(3):104-17. PubMed.
- Kaneko N, Nakamura A, Washimi Y, Kato T, Sakurai T, Arahata Y, Bundo M, Takeda A, Niida S, Ito K, Toba K, Tanaka K, Yanagisawa K. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(9):353-64. PubMed.
- Mc Donald JM, O'Malley TT, Liu W, Mably AJ, Brinkmalm G, Portelius E, Wittbold WM 3rd, Frosch MP, Walsh DM. The aqueous phase of Alzheimer's disease brain contains assemblies built from ∼4 and ∼7 kDa Aβ species. Alzheimers Dement. 2015 Apr 4; PubMed.
- Mattsson N, Rajendran L, Zetterberg H, Gustavsson M, Andreasson U, Olsson M, Brinkmalm G, Lundkvist J, Jacobson LH, Perrot L, Neumann U, Borghys H, Mercken M, Dhuyvetter D, Jeppsson F, Blennow K, Portelius E. BACE1 inhibition induces a specific cerebrospinal fluid β-amyloid pattern that identifies drug effects in the central nervous system. PLoS One. 2012;7(2):e31084. PubMed.
- Portelius E, Dean RA, Andreasson U, Mattsson N, Westerlund A, Olsson M, Demattos RB, Racke MM, Zetterberg H, May PC, Blennow K. β-site amyloid precursor protein-cleaving enzyme 1(BACE1) inhibitor treatment induces Aβ5-X peptides through alternative amyloid precursor protein cleavage. Alzheimers Res Ther. 2014;6(5-8):75. Epub 2014 Nov 17 PubMed.
- Pannee J, Törnqvist U, Westerlund A, Ingelsson M, Lannfelt L, Brinkmalm G, Persson R, Gobom J, Svensson J, Johansson P, Zetterberg H, Blennow K, Portelius E. The amyloid-β degradation pattern in plasma--a possible tool for clinical trials in Alzheimer's disease. Neurosci Lett. 2014 Jun 24;573:7-12. Epub 2014 May 4 PubMed.
- Bouter Y, Dietrich K, Wittnam JL, Rezaei-Ghaleh N, Pillot T, Papot-Couturier S, Lefebvre T, Sprenger F, Wirths O, Zweckstetter M, Bayer TA. N-truncated amyloid β (Aβ) 4-42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol. 2013 Aug;126(2):189-205. PubMed.
- Takeda K, Araki W, Akiyama H, Tabira T. Amino-truncated amyloid beta-peptide (Abeta5-40/42) produced from caspase-cleaved amyloid precursor protein is deposited in Alzheimer's disease brain. FASEB J. 2004 Nov;18(14):1755-7. PubMed.
- Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, Zetterberg H, Winblad B, Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer's disease. Acta Neuropathol. 2010 Aug;120(2):185-93. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.