With Amyloid Scan in Hand, Physicians Manage AD Differently
Quick Links
Amyloid PET scans have moved into clinical use, but questions remain about how much the technology benefits patients. Initial studies suggest amyloid imaging can sharpen diagnosis, and now new research from Italy adds to these data. In the Oct 31 JAMA Neurology, researchers led by Marina Boccardi at the University of Geneva reported that physicians in community clinics changed the diagnoses of about one-fourth of cognitively impaired patients based on amyloid scans. However, the physicians did not assign a diagnosis of Alzheimer’s disease to every patient who had brain amyloid, indicating they took other data into account as well. In addition, the doctors altered prescriptions in about one-third of the group after seeing scan data. This is one of the first large studies to examine what actually happens after amyloid scanning in clinical practice. The results jibe with previous, smaller studies, strengthening the argument that amyloid scanning does affect diagnosis and treatment. Nonetheless, these data leave unanswered the question of whether these changes improve a patient’s health and well-being enough to justify the scans’ hefty price tag.
“Although there may remain room for argument about cost, the findings of Boccardi and colleagues provide important insight into the real-world utility of amyloid PET,” Richard Caselli and Bryan Woodruff at the Mayo Clinic Arizona, Scottsdale, wrote in an accompanying editorial. Gil Rabinovici at the University of California San Francisco Memory and Aging Center agreed. “This study is encouraging in that it shows amyloid imaging has a clinical impact on diagnosis and patient management, and that clinicians are using the information in a nuanced way,” Rabinovici told Alzforum.
To date, only a handful of studies have addressed the question of how useful amyloid scanning might be in the clinic. Most have been small, often done at a single clinic, Rabinovici noted. The largest were sponsored by Avid Pharmaceuticals, the makers of florbetapir. They partly sponsored this study too, but had no role in the design, data management or interpretation, decision to publish, or review of the manuscript, according to the paper. In one prior study, a retrospective of 229 patients, physicians were asked to provide hypothetical treatment plans before and after considering amyloid scan data. Researchers reported that half the diagnoses changed in this somewhat artificial scenario (see Jan 2013 conference news). In a larger prospective study of 308 participants, Avid found that doctors changed diagnoses in about one-third of cases. However, these patients fared no better healthwise over the next year than did another 310 patients whose diagnosis was not based on scan data (see Aug 2015 conference news).
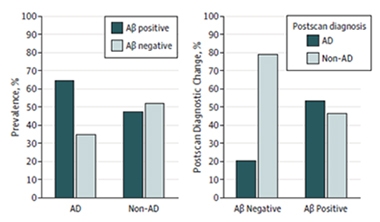
Negative Scans Sway Diagnosis Most.
PET scans revealed that about one-third of patients diagnosed with AD were amyloid-negative, while about half of patients with other diagnoses were amyloid-positive (left). Diagnoses for nearly all the Aβ-negative patients changed to non-AD (right). Only half the non-AD Aβ-positive patients were given a new diagnosis of AD. [Courtesy of Boccardi et al., © 2016 American Medical Association. All rights reserved.]
Boccardi and colleagues wanted to know how amyloid scans would perform in regular clinical practice. They recruited 228 participants with cognitive complaints and an uncertain diagnosis from 18 memory clinics in a region of northern Italy. Participants were 70 years old on average with an average MMSE score of 23, indicating mild impairment. The whole cohort underwent standardized clinical and neuropsychological testing and amyloid PET scanning. Other diagnostic tests, such as MRI scans, FDG PET, and cerebrospinal fluid collection, were done according to standard practice at each local clinic, and therefore varied across the group. Dementia experts at the clinics, who were neurologists or geriatricians, first developed a diagnosis and treatment plan without seeing the amyloid PET data. After looking at the scans, they revised their assessment.
Before the scans, 165 participants were diagnosed with AD, 37 with frontotemporal lobar degeneration (FTLD), and 26 with some form of subcortical disease, including cerebrovascular disease, dementia with Lewy bodies, corticobasal degeneration, and Parkinson’s disease dementia. In each of these cases, physicians were less than 85 percent sure of their diagnosis, in accordance with recommendations that amyloid scanning only be used to clarify uncertain diagnoses. About 14 percent to 27 percent of the patients who came to memory clinics ended up in this uncertain cohort, providing a rough estimate of how frequently amyloid scanning might be used in clinical practice.
The scans revealed that about one-third of people diagnosed with AD had brain amyloid below the cutoff for positivity, while about half of those with other diagnoses were above the cutoff (see graph above). After taking these data into account, physicians changed the diagnosis of most of the amyloid-negative AD patients, most commonly to cerebrovascular disease, FTLD, or depression. However, they stuck with an AD diagnosis for 12 patients who had a negative scan. On the other hand, only about half of amyloid-positive participants diagnosed with another disease were reclassified as having AD. Most of those had previously been diagnosed with FTLD. Overall, after scanning, 90 percent of those with an amyloid-positive scan ended up with an AD diagnosis, and 87 percent of those with a negative scan had other diagnoses. Moreover, physicians expressed more confidence in their diagnoses after seeing scan data.
Researchers praised the fact that clinicians did not simply equate an amyloid-positive scan with AD. “That’s appropriate. The scan should be just one data piece you use along with other clinical context to make a diagnosis,” Rabinovici said. He also liked the fact that clinicians put less weight on a positive scan than a negative one, recognizing that amyloid pathology can occur in other disorders. Nevertheless, the 12 amyloid-negative patients maintained their AD diagnosis because they fit the profile of Alzheimer’s extremely well, Boccardi noted. These patients might have had false negative scans, or they might have suspected non-Alzheimer’s pathology (SNAP), she suggested. Analysis of the collected CSF for disease biomarkers might shed additional light on their pathology.
The scan data affected disease management as well. Doctors prescribed an acetylcholinesterase inhibitor or memantine to about two-thirds of the amyloid-positive patients who were not previously taking these drugs, and discontinued them for about one-third of amyloid-negative patients. The physicians also changed other medications, such as antidepressants, antipsychotics, and anxiety drugs, in about 10 percent of the cohort after scanning.
The study did not address whether these medication changes affected the health of the patients. Acetylcholinesterase inhibitors and memantine have been found to be harmful in FTLD patients (see Mendez et al., 2007; Arciniegas and Anderson, 2013; Boxer et al., 2013).
The large ongoing IDEAS study in the United States, which will comprise 18,000 scans, is tackling this issue by comparing hospitalization and other medical outcomes in patients of uncertain diagnosis who undergo scanning versus those who do not (see Apr 2015 news). The Centers for Medicare and Medicaid Services (CMS) pays for these scans, but will not cover amyloid scanning outside of this trial until the technology has been deemed cost-effective (see Jul 2013 conference news). IDEAS will also examine a range of other outcomes, such as whether patients follow the doctor’s treatment recommendations, and whether they make other life changes such as financial planning or joining a clinical trial, noted Rabinovici, who leads the study.
A similar study of amyloid scan benefits, AMYPAD, is currently gearing up in Europe, with a goal of evaluating about 6,000 brain scans in conjunction with the EPAD project.
In addition, the Italian authors did not attempt to quantify whether the benefits of amyloid scanning justify the cost, which runs about €2,000 in Italy and between $3,000 and $6,000 in the United States. Future studies by the group will compare the diagnostic benefit of amyloid scanning with cerebrospinal fluid biomarker analysis, which is much cheaper, Boccardi told Alzforum. In the past, CSF measurements varied too much between different labs to be reliable, but these markers are approaching standardization (see Oct 2015 news).
Boccardi is running additional analyses of her data to study other aspects of the clinical use of amyloid scans, including determining which patients benefit most from them. The Alzheimer’s field has established Appropriate Use Criteria to help select the best candidates for these scans (see Jan 2013 news). Boccardi will mine her data to find out if these criteria hold up in clinical practice.—Madolyn Bowman Rogers
References
News Citations
- HAI—Amyloid Imaging in the Clinic: New Guidelines and Data
- Amyloid Scans in the Clinic: Seeing Is Believing?
- $100M IDEAS: CMS Blesses Study to Evaluate Amyloid Scans in Clinical Practice
- Coverage Denial For Amyloid Scans Riles Alzheimer’s Community
- Not Sexy but Oh-So-Important: Committee Blesses Way to Measure CSF Aβ
Paper Citations
- Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007 Jan;15(1):84-7. PubMed.
- Arciniegas DB, Anderson CA. Donepezil-induced confusional state in a patient with autopsy-proven behavioral-variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2013;25(3):E25-6. PubMed.
- Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, Mendez M, Kerwin D, Lerner A, Wu CK, Koestler M, Shapira J, Sullivan K, Klepac K, Lipowski K, Ullah J, Fields S, Kramer JH, Merrilees J, Neuhaus J, Mesulam MM, Miller BL. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013 Feb;12(2):149-56. PubMed.
External Citations
Further Reading
Primary Papers
- Boccardi M, Altomare D, Ferrari C, Festari C, Guerra UP, Paghera B, Pizzocaro C, Lussignoli G, Geroldi C, Zanetti O, Cotelli MS, Turla M, Borroni B, Rozzini L, Mirabile D, Defanti C, Gennuso M, Prelle A, Gentile S, Morandi A, Vollaro S, Volta GD, Bianchetti A, Conti MZ, Cappuccio M, Carbone P, Bellandi D, Abruzzi L, Bettoni L, Villani D, Raimondi MC, Lanari A, Ciccone A, Facchi E, Di Fazio I, Rozzini R, Boffelli S, Manzoni L, Salvi GP, Cavaliere S, Belotti G, Avanzi S, Pasqualetti P, Muscio C, Padovani A, Frisoni GB, Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Working Group. Assessment of the Incremental Diagnostic Value of Florbetapir F 18 Imaging in Patients With Cognitive Impairment: The Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Study. JAMA Neurol. 2016 Dec 1;73(12):1417-1424. PubMed.
- Caselli RJ, Woodruff BK. Clinical Impact of Amyloid Positron Emission Tomography-Is It Worth the Cost?. JAMA Neurol. 2016 Oct 31; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.