Wanted: Fluid Biomarkers for CAA, ARIA
Quick Links
How can researchers make amyloid immunotherapy safer? At the Alzheimer’s Association International Conference, held July 16-20 in Amsterdam, several speakers linked the amyloid-related imaging abnormalities that are the worst side effects of immunotherapy to cerebrovascular amyloid. ARIA may be an inflammatory reaction to cerebral amyloid angiopathy, they proposed (see Part 6 of this series). If so, screening patients for CAA could help gauge their risk of ARIA and guide treatment decisions. The problem? There are no reliable biomarkers for CAA.
- Fluid biomarkers for CAA could help predict the risk of ARIA.
- Early candidates include complement protein C3 and the vascular protein medin.
- For ARIA itself, nominees include Aβ auto-antibodies and plasma NfL.
Researchers contacted by Alzforum said there are, as yet, few studies in this area, but that research is heating up. Speakers at AAIC proposed some candidate markers. Meanwhile, pharma scientists are interested in developing fluid biomarkers for ARIA, to help catch the condition earlier and cut down on the expense of MRI scans.
“Biomarkers for CAA and ARIA are critically needed right now,” Suzanne Schindler of Washington University in St. Louis told Alzforum. Mathias Jucker of the University of Tübingen, Germany, agreed this should be a research priority. He noted that early studies of immunotherapy in amyloidosis mouse models had suggested a link with CAA (e.g., Pfeifer et al., 2002; Burbach et al., 2007), but because of the failure of the first such trials, research funding and interest waned. “This is a good example of why basic research is important,” Jucker wrote to Alzforum. “Had we all continued to work on this topic over the last 10 years, we would probably know much more today about the mechanisms of ARIA.”
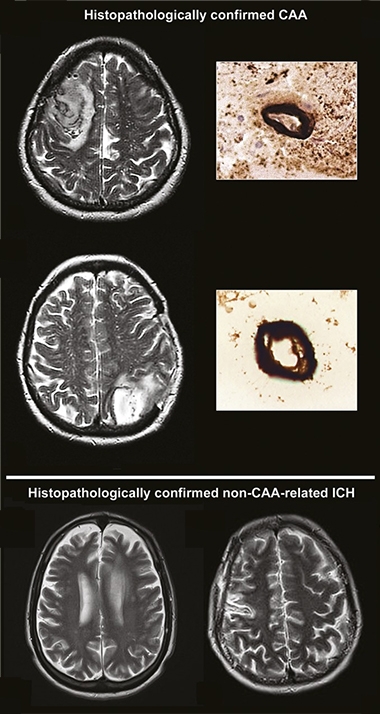
CAA on MRI. Currently, clinicians diagnose CAA on MRI scans, where excess fluid around blood vessels shows up as bright white areas (top, vessels in cross-section), distinguishing it from intracerebral hemorrhages that occur without CAA (bottom). [Courtesy of David Werring, University College London.]
Detecting CAA in Living Brains
For now, clinicians look for CAA by assessing white-matter damage on MRI scans. However, this is unreliable. “Radiographic imaging markers of CAA have been clearly demonstrated to have insufficient specificity and sensitivity,” Fabrizio Piazza of the University of Milano-Bicocca, Italy, told Alzforum.
Scientists are searching for fluid biomarkers, and have a few leads. In Amsterdam, Cynthia Lemere of Brigham and Women’s Hospital, Boston, proposed certain complement proteins as candidate markers of vascular amyloid. In particular, the concentration of the complement factor C3 in the blood could flag the presence of CAA in the brain, she noted.
Why is this? Cristian Lasagna-Reeves of Indiana University School of Medicine, Indianapolis, found that when mice had cerebrovascular amyloid, their brain endothelial cells triggered astrocytes to become neurotoxic, releasing C3 in the process (Taylor et al., 2022). In a study of 55 people with MCI, elevated serum C3 helped distinguish the 16 with CAA from the 39 without, with every 0.1 unit/mL increase in C3 bumping up their odds of having CAA by 1.4 times (Saito et al. 2022).
In addition, Lemere discussed a potentially more exotic CAA marker, small vesicles known as migrasomes. These membrane blobs are left behind by migrating macrophages. Last month, scientists led by Wei Cai of Sun Yat-Sen University, Guangzhou, China, reported that cultured macrophages exposed to vascular amyloid spit out migrasomes stuffed with the glycoprotein CD5L. CD5L modulates macrophage activity and is part of the body’s defense against infections. However, this protein attaches to vascular amyloid and triggers the complement cascade, activating the C5b-9 membrane attack complex and damaging the blood-brain barrier in the process (Hu et al., 2023). CD5L and C5b-9, as well as migrasomes themselves, may be useful biomarkers of CAA, Lemere suggested.
Jonas Neher of the German Center for Neurodegenerative Diseases in Tübingen previously nominated medin, a protein fragment that aggregates into a vascular amyloid that promotes CAA, as a potential marker (Nov 2022 news). His group has developed highly specific antibodies against medin and is using these to test its association with CAA, Neher told Alzforum. He has also collected CSF from mouse models with and without CAA, and will compare their protein profiles to turn up more candidate markers. Human validation will be challenging, however, because so far there is no large collection of CSF or blood samples from patients with neuropathologically diagnosed CAA, Neher said.
Another recent paper from Marcel Verbeek of Radboud University Medical Center, Nijmegen, The Netherlands, associated a dearth of matrix metalloproteinases MMP-2 and MMP-14 in the CSF with both sporadic CAA and the genetic form caused by the Dutch APP mutation (Vervuurt et al., 2023). CAA was previously linked to heightened MMP activity; it is unclear what the mechanism for lowered MMP might be (Jung et al., 2003; Zhao et al., 2015; Tanskanen et al., 2011).
There are some large-scale efforts to find biomarkers of CAA and vascular damage, and these might provide the needed fluid samples for other studies. Piazza runs the Inflammatory CAA and AD Biomarkers International Network (iCAβ). This study enrolls people with CAA-related inflammation who develop ARIA-like edema, and collects their cerebrospinal fluid and blood. The goal is to identify diagnostic and prognostic markers of CAA and ARIA.
Similarly, the MarkVCID project led by Steven Greenberg at Massachusetts General Hospital, Boston, looks for biomarkers that detect vascular contributions to cognitive impairment and dementia (Mar 2017 news). Though not specifically aimed at CAA, the research may fish out such biomarkers.
Fluid Biomarkers of ARIA-E?
Despite the interest in CAA biomarkers, Piazza cautioned that CAA is only a risk factor for ARIA. Under the right conditions, vascular amyloid can trigger inflammation that leads first to the edema known as ARIA-E, and then to microhemorrhages known as ARIA-H. “The real urgent international research priority is biomarkers for ARIA-E,” Piazza said. Not only would quicker detection of ARIA-E improve safety, but biomarkers could deepen researchers’ understanding of how amyloid immunotherapy works, he added.
Because auto-antibodies to Aβ are associated with CAA-related inflammation, Piazza believes they might identify people at the highest risk for ARIA-E (Piazza et al., 2013). In addition, Piazza suggested that monitoring the levels of such auto-antibodies during immunotherapy, and pausing treatment if they rise, could improve safety.
Kaj Blennow and Henrik Zetterberg of the University of Gothenburg think plasma NfL might be a useful ARIA-E biomarker. They noted that talks in Amsterdam showed that this marker of neurodegeneration first rose and then fell in people treated with donanemab (Jul 2023 conference news). This peak might reflect ARIA, which tends to occur shortly after starting immunotherapy, they speculated. If so, testing plasma NfL levels at baseline and every two weeks could provide an early warning. If NfL spiked, doctors would order an MRI scan. Because plasma NfL can be tested on automated platforms, monitoring for ARIA this way could be more frequent, and much cheaper, than with repeated MRIs, Blennow said.
Ultimately, however, research into ARIA biomarkers will depend on the companies that developed anti-amyloid antibodies, because they have access to the crucial clinical trial samples, Neher noted. In Amsterdam, pharma scientists said this research is a priority for them. Katherine Dawson of Biogen said the company is looking at risk factors that might predict ARIA’s occurrence, for example blood-brain-barrier damage. Roche Diagnostics is trying to develop blood tests for ARIA, investigating molecules such as homocysteine to see if they predict risk. Elevated homocysteine is associated with blood clots and vascular damage. “This is high on the radar screen at Roche,” Rachelle Doody of Roche said.—Madolyn Bowman Rogers
References
News Citations
- Is ARIA an Inflammatory Reaction to Vascular Amyloid?
- Meddling Medin: A Vascular Amyloid That Promotes CAA?
- Consortium to Seek Biomarkers for Vascular Cognitive Impairment
- Donanemab Data Anchors Upbeat AAIC
Therapeutics Citations
Paper Citations
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002 Nov 15;298(5597):1379. PubMed.
- Burbach GJ, Vlachos A, Ghebremedhin E, Del Turco D, Coomaraswamy J, Staufenbiel M, Jucker M, Deller T. Vessel ultrastructure in APP23 transgenic mice after passive anti-Abeta immunotherapy and subsequent intracerebral hemorrhage. Neurobiol Aging. 2007 Feb;28(2):202-12. PubMed.
- Taylor X, Cisternas P, Jury N, Martinez P, Huang X, You Y, Redding-Ochoa J, Vidal R, Zhang J, Troncoso J, Lasagna-Reeves CA. Activated endothelial cells induce a distinct type of astrocytic reactivity. Commun Biol. 2022 Mar 29;5(1):282. PubMed.
- Saito S, Yamashiro T, Yamauchi M, Yamamoto Y, Noguchi M, Tomita T, Kawakami D, Shikata M, Tanaka T, Ihara M. Complement 3 Is a Potential Biomarker for Cerebral Amyloid Angiopathy. J Alzheimers Dis. 2022;89(1):381-387. PubMed.
- Hu M, Li T, Ma X, Liu S, Li C, Huang Z, Lin Y, Wu R, Wang S, Lu D, Lu T, Men X, Shen S, Huang H, Liu Y, Song K, Jian B, Jiang Y, Qiu W, Liu Q, Lu Z, Cai W. Macrophage lineage cells-derived migrasomes activate complement-dependent blood-brain barrier damage in cerebral amyloid angiopathy mouse model. Nat Commun. 2023 Jul 4;14(1):3945. PubMed.
- Vervuurt M, de Kort AM, Jäkel L, Kersten I, Abdo WF, Schreuder FH, Rasing I, Terwindt GM, Wermer MJ, Greenberg SM, Klijn CJ, Kuiperij HB, Verbeek MM. Decreased ratios of matrix metalloproteinases to tissue-type inhibitors in cerebrospinal fluid in sporadic and hereditary cerebral amyloid angiopathy. Alzheimers Res Ther. 2023 Jan 30;15(1):26. PubMed.
- Jung SS, Zhang W, Van Nostrand WE. Pathogenic A beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem. 2003 Jun;85(5):1208-15. PubMed.
- Zhao L, Arbel-Ornath M, Wang X, Betensky RA, Greenberg SM, Frosch MP, Bacskai BJ. Matrix metalloproteinase 9-mediated intracerebral hemorrhage induced by cerebral amyloid angiopathy. Neurobiol Aging. 2015 Nov;36(11):2963-71. Epub 2015 Jul 16 PubMed.
- Tanskanen M, Myllykangas L, Saarialho-Kere U, Paetau A. Matrix metalloproteinase-β19 expressed in cerebral amyloid angiopathy. Amyloid. 2011 Mar;18(1):3-9. PubMed.
- Piazza F, Greenberg SM, Savoiardo M, Gardinetti M, Chiapparini L, Raicher I, Nitrini R, Sakaguchi H, Brioschi M, Billo G, Colombo A, Lanzani F, Piscosquito G, Carriero MR, Giaccone G, Tagliavini F, Ferrarese C, Difrancesco JC. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: Implications for Amyloid-Modifying Therapies. Ann Neurol. 2013 Feb 11; PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Radboud University Nijmegen Medical Centre
The interest in research on CAA has grown exponentially since the results of multiple clinical trials with anti-Aβ immunotherapy showed that these novel therapies are associated with the development of ARIA. In a sizeable percentage of patients, immunotherapy is complicated by the occurrence of either ARIA-E (vasogenic edema and extravasated fluid) or ARIA-H (microhemorrhages and hemosiderosis). ARIA may lead to rapidly progressive (or subacute) behavioral-cognitive changes, headache, seizures, and focal neurological deficits.
ARIA is closely related to CAA for several reasons: 1) ARIA resembles the abnormalities seen in an aggressive form of CAA, i.e., CAA-related inflammation (CAA-ri), both clinically and radiologically (Sperling et al., 2011); (2) Patients with CAA, CAA-ri, and ARIA more often have the APOE-ε4 genotype (Salloway et al., 2014); (3) Neuropathological studies have demonstrated that following immunotherapy, plaque-associated Aβ can be removed, but CAA remains and may even increase (Sakai et al., 2014); (4) In transgenic mouse models of AD, anti-Aβ immunotherapy resulted in an increase in CAA-associated hemorrhage (Pfeifer et al., 2002).
A biomarker to predict the development of ARIA could help in selecting patients for immunotherapy and prevent the serious side effects of these treatments, thus increasing the success rate of immunotherapy in AD.
In 2022, Charidimou et al. proposed the Boston criteria version 2.0 for cerebral amyloid angiopathy (Charidimou et al., 2022). Whereas brain biopsy is rarely performed in patients, these criteria lean on MRI-based detection of hemorrhagic and non-hemorrhagic consequences of CAA. As a result, according to recently proposed pathophysiological framework of CAA (Koemans et al., 2023), diagnosis of CAA using these criteria is limited to detection of CAA in stages 3 and 4. Indeed, as we recently demonstrated, there is a substantial underestimation of the true prevalence of CAA in humans when comparing the neuroradiology-based diagnosis of CAA to the gold standard of a neuropathological diagnosis (Jäkel et al., 2021).
In several publications, we have demonstrated that cerebrospinal fluid (CSF) concentrations of the frequently studied Aβ40 and Aβ42 proteins are decreased in CAA, which has been confirmed by other researchers (Verbeek et al., 2009; Charidimou et al., 2018). Subsequently, we extended these findings to the observation that CSF concentrations of Aβ40 and Aβ42 proteins are also clearly decreased in the presymptomatic stages of Dutch-type hereditary CAA, and continue to decrease in the symptomatic stage and therefore reflect the first stages of CAA evolution (van Etten et al., 2017). More recently, we extended these biomarker observations to similarly decreased levels of CSF Aβ38 and Aβ43 (De Kort et al., 2023).
Taken together, we conclude that CSF biomarkers, and in particular isoforms of Aβ shorter than 42 amino acids, are specific diagnostic biomarkers of CAA. Therefore, quantification of the levels of CSF Aβ proteins might be suitable as biomarkers to identify those individuals with a high CAA load, and who should be excluded from anti-Aβ immunotherapies to avoid the harmful side effects of ARIA, and possibly increase the clinical efficacy of these interventions.
References:
Sperling RA, Jack CR, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011 Jul;7(4):367-85. PubMed.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014 Jan 23;370(4):322-33. PubMed.
Sakai K, Boche D, Carare R, Johnston D, Holmes C, Love S, Nicoll JA. Aβ immunotherapy for Alzheimer's disease: effects on apoE and cerebral vasculopathy. Acta Neuropathol. 2014 Dec;128(6):777-89. Epub 2014 Sep 7 PubMed.
Jäkel L, De Kort AM, Klijn CJ, Schreuder FH, Verbeek MM. Prevalence of cerebral amyloid angiopathy: A systematic review and meta-analysis. Alzheimers Dement. 2021 May 31; PubMed.
Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002 Nov 15;298(5597):1379. PubMed.
Charidimou A, Boulouis G, Frosch MP, Baron JC, Pasi M, Albucher JF, Banerjee G, Barbato C, Bonneville F, Brandner S, Calviere L, Caparros F, Casolla B, Cordonnier C, Delisle MB, Deramecourt V, Dichgans M, Gokcal E, Herms J, Hernandez-Guillamon M, Jäger HR, Jaunmuktane Z, Linn J, Martinez-Ramirez S, Martínez-Sáez E, Mawrin C, Montaner J, Moulin S, Olivot JM, Piazza F, Puy L, Raposo N, Rodrigues MA, Roeber S, Romero JR, Samarasekera N, Schneider JA, Schreiber S, Schreiber F, Schwall C, Smith C, Szalardy L, Varlet P, Viguier A, Wardlaw JM, Warren A, Wollenweber FA, Zedde M, van Buchem MA, Gurol ME, Viswanathan A, Al-Shahi Salman R, Smith EE, Werring DJ, Greenberg SM. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022 Aug;21(8):714-725. PubMed.
Koemans EA, Chhatwal JP, van Veluw SJ, van Etten ES, van Osch MJ, van Walderveen MA, Sohrabi HR, Kozberg MG, Shirzadi Z, Terwindt GM, van Buchem MA, Smith EE, Werring DJ, Martins RN, Wermer MJ, Greenberg SM. Progression of cerebral amyloid angiopathy: a pathophysiological framework. Lancet Neurol. 2023 Jul;22(7):632-642. Epub 2023 May 23 PubMed.
Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009 Aug;66(2):245-9. PubMed.
Charidimou A, Friedrich JO, Greenberg SM, Viswanathan A. Core cerebrospinal fluid biomarker profile in cerebral amyloid angiopathy: A meta-analysis. Neurology. 2018 Feb 27;90(9):e754-e762. Epub 2018 Jan 31 PubMed.
van Etten ES, Verbeek MM, van der Grond J, Zielman R, van Rooden S, van Zwet EW, van Opstal AM, Haan J, Greenberg SM, van Buchem MA, Wermer MJ, Terwindt GM. β-Amyloid in CSF: Biomarker for preclinical cerebral amyloid angiopathy. Neurology. 2017 Jan 10;88(2):169-176. Epub 2016 Nov 30 PubMed.
De Kort AM, Kuiperij HB, Marques TM, Jäkel L, van den Berg E, Kersten I, van Berckel-Smit HE, Duering M, Stoops E, Abdo WF, Rasing I, Voigt S, Koemans EA, Kaushik K, Warren AD, Greenberg SM, Brinkmalm G, Terwindt GM, Wermer MJ, Schreuder FH, Klijn CJ, Verbeek MM. Decreased Cerebrospinal Fluid Amyloid β 38, 40, 42, and 43 Levels in Sporadic and Hereditary Cerebral Amyloid Angiopathy. Ann Neurol. 2023 Jun;93(6):1173-1186. Epub 2023 Feb 20 PubMed. Correction.
Make a Comment
To make a comment you must login or register.