Drug Trials in Frontotemporal Dementia: Can Field Push Forward Together?
Quick Links
On March 31 and April 1, 95 scientists from 23 companies, 19 academic institutions, as well as public and private funding and related organizations gathered in Washington, D.C., for the Frontotemporal Dementia Study Group’s fourth workshop. While the city’s cherry blossoms bloomed riotously outside, the scientists huddled in a hotel basement room with advocates, family members, and people with FTD. They took stock of how far the field has come in its quest to understand this heterogeneous set of diseases, and what tools they most urgently need to develop medications to treat or prevent them. Importantly, the group got an opportunity to quiz three leading regulatory scientists for advice on how to develop drugs for them (see Part 3 of this series). Disorders across the FTD spectrum encompass behavioral variant FTD, three different forms of primary progressive aphasia, progressive supranuclear palsy, corticobasal degeneration, and FTD with motor neuron disease. Together they afflict 50,000 to 60,000 people and their families in the United States. No approved treatments exist.
Overall, eight hours of meetings imparted four lessons:
- The basic science on FTDs is creating unprecedented momentum.
- The setbacks of Alzheimer’s drug development hold valuable lessons.
- Biomarkers are beginning to emerge, but much more work is needed.
- The Food and Drug Administration and European Medicines Agency urge rigorous science that links biomarker change to meaningful clinical outcomes.
Emphasis on openness and data sharing resonated throughout. The vision of a shared infrastructure platform for Phase 1/2 FTD trials was briefly floated, but while the regulators in the room welcomed the idea, pharmaceutical companies appeared not quite ready to embrace this deeper level of collaboration.
“FTD is a young field. We are encountering a friendly and open world, with lots of advice from Alzheimer’s and Parkinson’s,” said Susan Dickinson of the Association for Frontotemporal Dementia in Radnor, Pennsylvania. “You have a fantastic opportunity here. We at the agency are enthusiastic about greater attention to these horrible diseases,” William Dunn of the Food and Drug Administration told the assembled audience. “It’s amazing how fast the FTD field has gone from description of syndromes to molecular pathophysiology, discovering neuroimaging metrics and biomarkers. This happened much faster than the decades it took the Alzheimer’s field to get to where FTD is now,” said Geoffrey Kerchner of Genentech in South San Francisco, California, which is developing a therapeutic tau antibody and a tau PET tracer. “Let’s acknowledge the importance of this meeting. These kinds of partnerships are critical to achieve our goals,” said Brad Dickerson, who runs a large FTD clinic at Massachusetts General Hospital. See below for a detailed summary.
The AFTD and the National Institute of Neurologic Disorders and Stroke jointly sponsored the conference. Dickinson noted that despite broad international consensus on diagnostic criteria for FTDs, most physicians still do not have these diseases on their list of potential diagnoses for middle-aged people who present with marital strife, disordered behavior, or failing language. That said, attention in research and industry has picked up tremendously in the past few years. For its part, AFTD alone has increased research funding from $35,000 in 2005 to $3 million in 2016, and a handful of other philanthropies have sprung up to support specific FTD subtypes. Together these groups have assembled a community of patients and gene carriers who want to participate in trials. They also want a say in decision-making along the way. “Patients and their families are here today, as well. They are ready to do their part in the work,” Dickinson said, adding that every board member at AFTD cares, or has cared, for a relative with FTD.
The organizers did not come empty-handed. Chief among the new funding opportunities they brought to the meeting was the $5 million FTD Biomarkers Initiative. This invites proposals to discover new, or develop existing, biomarkers for any disease across the FTLD spectrum. Company scientists are welcome to apply, too, said Nadine Tatton of AFTD, which co-funds drug discovery grants with the Alzheimer’s Drug Discovery Foundation (ADDF). For its part, the NINDS recently announced a funding opportunity for a multi-institutional center without walls to identify and validate molecular mechanisms that contribute to tau pathogenesis and associated neurodegeneration in FTD. This U54 grant addresses the top basic science recommendation from the NIH ADRD FTD working group. The winning proposal will use existing infrastructures, and feature ample data and resource-sharing as well as collaboration with non-governmental organizations and philanthropy, said NINDS’ Margaret Sutherland. In addition, NINDS announced PAR 16-122, a grant focused on parkinsonism that could support biomarker and clinical data collection for progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), two forms of FTD that lie on the parkinsonian end of the FTLD spectrum. At the NINDS Alzheimer’s and Related Diseases Summit, held at NIH earlier in the week, Ron Petersen of the Mayo Clinic, Rochester, Minnesota, estimated that about $161 million of the $991 million overall federal ADRD research budget would be spent on the related diseases, which include FTD (see Apr 2016 conference news).
The Challenge: A Varied, Complex Biology
The heterogeneity of the frontotemporal dementia spectrum makes drug development a great challenge, said David Knopman of the Mayo Clinic in Rochester. Its two main pathologic lesions—tauopathy and TDP-43 proteinopathy—each occur as several different subtypes, and debate continues about what form of the two respective proteins constitutes the toxic species. Mutations in three main genes—tau, progranulin, C9ORF72—plus several minor ones cause frontotemporal dementia. The genes map somewhat consistently to particular pathologies, though a large proportion of FTD appears sporadic.

Frontotemporal dementias all feature neurodegeneration in the front and side of the brain (left); beyond that, their clinical expression, genetic origin, and underlying protein pathologies are highly heterogeneous. [Courtesy of David Knopman.]
Perhaps most puzzling for trialists: Most FTDs show no clear link between their clinical picture and a given protein pathology. This means that when a patient comes to an FTD clinic, even experienced diagnosticians do not know if they are dealing with tau or TDP-43, or indeed FUS, yet another molecular pathology in FTLD. It also means that a therapy trial cohort enrolled by way of clinical criteria and MRI, but without a biomarker that ascertains an underlying molecular pathology, might constitute a mix of people with tau, TDP-43, or FUS pathology. This may be workable for neuroprotective or other drugs that target common mechanisms of neurodegeneration, but for tau-based drugs, it could dilute the number of people in the cohort who are likeliest to respond to the therapy. For example, the bvFTD cohort of the current TRx0237 Phase 3 study was enrolled in this way.
bvFTD is indeed the enfant terrible of the lot. It is the most common form of FTD, but a full 15 etiologies have been described to underlie its clinical presentation, said Bill Seeley from the University of California, San Francisco. A more typical example is primary progressive aphasia. This language disease can be sporadic or caused by progranulin or tau mutations, and feature either TDP-43 or tau pathology. An exception is PSP, a sporadic disease that almost always features tau pathology. This clear clinic-pathological relationship, combined with the disease’s rapid progression and the existence of a serviceable rating scale, has made PSP patients sought-after participants for a growing number of small trials of candidate drugs targeting tau.

How do you run trials in this? Most clinical types of FTD can have one of several underlying pathologies. Mutations in a given gene lead to different clinical diseases. [Courtesy of David Knopman and Dennis Dickson.]
This heterogeneity, compounded by the small number of available patients, means that successful therapy evaluation will require biomarkers for a diagnosis and for target engagement for each molecular subtype of FTD.
The Status: Work in Models to Find Molecular Mechanisms
On the tau side of the FTLD spectrum, the heterogeneity is apparent in distinct cellular pathologies and rates of progression between one person’s disease and another’s. It may stem in part from the manifold post-translational modifications tau undergoes and in part from tau’s ability to form strains. Strains are structural variants of tau that cause a particular form of tau pathology. They can be isolated from diseased human brain and faithfully passaged between cells and mice. “Strains are the building blocks of tauopathy,” said Marc Diamond of UT Southwestern Medical Center in Dallas.
The TDP-43 proteinopathies in particular have experienced a surge of interest. Of the 1,837 papers published in PubMed since the TARDBP gene was cloned in 1995, 1,825 appeared since Manuela Neumann and Tetsuaki Arai implicated TDP-43 in neurodegeneration (Oct 2006 news); 1,000 have appeared in the last four years alone. (Not all these papers are on TDP-43 alone, some merely mention it.)
This nuclear RNA-binding protein regulates its own production and, for poorly understood reasons, it ends up in the cytoplasm where it accumulates in a variety of structures. “We must, for therapeutic purposes, understand what causes this mislocalization,” Leonard Petrucelli of Mayo Clinic in Jacksonville, Florida, told the assembled FTSG audience. Nuclear export and import has become a prominent theme in understanding TDP-43 aggregation.
The protein’s C-terminus hosts mutations causing ALS and some cases of FTLD. Functionally, this end mediates formation of stress granules and cytoplasmic aggregates. The protein’s N-terminus is thought to regulate multiple different aspects of the lives of hundreds of different target RNAs, from pre-mRNA splicing to microRNA processing, non-coding and long non-coding RNA metabolism, as well as mRNA transport, stability, and translation (for open-access review, see Ratti and Buratti, 2016).
Researchers have generated a host of TDP-43 mouse models to study whether gain-of-function versus loss-of-function underlie toxicity (see Alzforum Research Models Database). Biomarkers are farther behind. No PET tracer is yet in sight, though the Swiss biotech company AC Immune and Biogen on April 18 announced a partnership to develop one (see company press release). Current CSF or blood assays work poorly, in part because peripheral cells produce more TDP-43 than the brain. This makes it difficult to define how much of a given concentration in a fluid sample represents brain pathology, said Kaj Blennow of the University of Gothenburg, Sweden.
Like TDP-43, C9ORF72 has taken the FTD research community by storm. It has generated 684 PubMed entries since Rosa Rademakers and Brian Traynor discovered, in 2011, hexanucleotide repeat expansion in this gene. On April 18 at the American Academy of Neurology’s annual meeting in Vancouver, British Columbia, these two neurogeneticists received the 2016 Potamkin Prize for Research in Pick’s, Alzheimer’s, and Related Diseases (see Apr 2016 news).
C9ORF72 expansions are the most common genetic cause of FTD, ALS, or ALS-FTD. Mutation carriers have aggregated TDP-43 inclusions in their central nervous system, and the C9 expansion has been linked to mislocalization and aggregation of TDP-43. “How that happens is a key question in the field these days,” said Petrucelli. More broadly, the abiding research question for C9ORF72, just like for TDP-43, is whether the DNA expansion is toxic by way of a loss of the gene’s normal function, or gain of toxic function. The cellular function of the C9ORF72 protein is poorly understood, but it is implicated in endosomal/autophagy-related membrane trafficking and thought to play a role in innate immunity. Even so, researchers do not currently favor haploinsufficiency as the main pathogenic mechanism of C9ORF72 diseases. A toxic gain of function could result when RNA transcripts of the C9 repeats aggregate into foci that entrap cellular RNA-binding proteins. It could also arise from defects in transport between nuclear and cytoplasmic factors, or from translation of the DNA repeat expansions into aggregating dipeptide repeat proteins that could damage proteasome degradation or cause ER stress, Petrucelli told the audience.
Clinically, dipeptide repeat pathology has been placed earlier in the C9ORF72 disease process than TDP-43 inclusions, but attempts at staging disease remain in their infancy. Three mouse models, but no fluid or imaging biomarkers, are available for study (see Alzforum Research Models). For an open-access review of C9ORF72, see Todd and Petrucelli, 2016.
The Status: Work in Humans to Chart Evolution of FTD
Parallel to research in mice and cell-based models, initiatives are underway on both sides of the Atlantic to characterize the natural history of FTD in human cohorts. In Alzheimer’s and Parkinson’s, studies such as DIAN, AIBL, ADNI, and PPMI are showing how biomarkers change over time and how to quantify disease progression. This has helped galvanize industry, and they want the same for FTD. “We have to have longitudinal human data. That is our key ‘ask’ to be able to run successful trials,” Philipp von Rosenstiel at Biogen in Cambridge, Massachusetts, told the FTSG. Biogen is developing a preclinical anti-tau antibody.

This sequence of events is how scientists theorize FTD might develop. GENFI and LEFFTDS are generating empirical data to test this staging model. [Courtesy of Jonathan Rohrer, UCL.]
The most advanced project is the Genetic FTD Initiative. Inspired by DIAN for autosomal-dominant Alzheimer’s, GENFI started in 2012 as a multicenter consortium to gather carriers of pathogenic mutations in the progranulin, tau, and C9ORF72 genes and their relatives into a cohort. The goal was to track their disease process with standardized assessments of biomarkers and clinical symptoms, including standardized cognitive tests across European languages. “With GENFI1, a key point was to see if we would be able to do this,” Jonathan Rohrer of University College London told the audience. They were, and the result was a standing project among 13 centers in Europe and Canada that is beginning to order biomarkers across preclinical and clinical stages of disease. Cross-sectional data from a data freeze are published (see Nov 2014 conference news; Rohrer et al., 2015). When GENFI-1 ended in spring 2015 and GENFI-2 started, the scientists had collected baseline data on 365 participants and 148 follow-up visits, Rohrer said.
April 2016 marks year two of GENFI-2, which has expanded to 27 centers in Europe and Canada and is currently enrolling for a target of 600 participants coming for three visits. Some GENFI-1 participants are continuing into this next phase of the project. Thus far, 303 are enrolled, with the largest fraction being progranulin families. Because GENFI-2 can build on the infrastructure and protocol of GENF1, the researchers can now focus more on trial preparation. Besides creating a trial-ready cohort, their goals include trying to pin down biomarkers that indicate the best time to interfere therapeutically, as well as progression markers to measure whether a drug might be working, Rohrer said. GENFI will make its data broadly available for the field to study, Rohrer promised.
Separately, in cities across the United States plus Vancouver and Toronto, two interconnected initiatives started up in spring 2015 to accomplish much the same goals. Called ARFTL and LEFFTDS, they are described in detail in this Dec 2014 conference story.
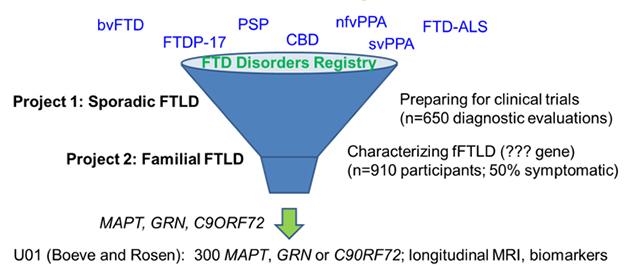
In its twin efforts to prepare participants for upcoming therapeutic trials and discover new familial cases, the ARFTL initiative welcomes people with all forms of FTD, both sporadic and hereditary. [Courtesy of Adam Boxer, UCSF.]
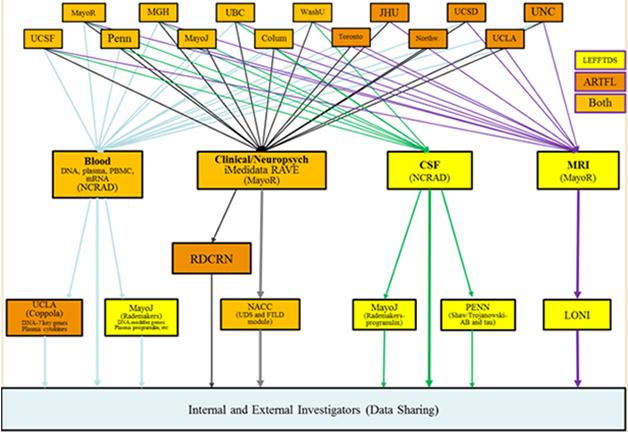
This image captures how participating institutions coordinate the components of the ARTFL and LEFFTDS FTD research initiatives. Different centers house repositories for storage of blood and CSF samples, MRI scans, and clinical data. [Courtesy of Brad Boeve, Mayo Clinic, Rochester.]
ARFTL’s leader, Adam Boxer of the University of California, San Francisco, also chairs the Frontotemporal Dementia Treatment Study Groups' steering committee. In D.C., he told the audience that ARTFL is broader than GENFI and LEFFTDS in that it aims to find out how best to run therapy trials not just in genetic but also in sporadic forms of FTD. These still constitute the majority of cases, and in most of those clinicians do not have the advantage of knowing which protein pathology drives a given person’s disease.
Boxer noted that one big priority was to increase the number of participants for trials across FTD with the help of a registry. After initially exploring the existing Rare Clinical Diseases Research Network (RCDRN) for this purpose, the investigators opted instead to custom-build an online tool called the FTD Disorders Registry. Led by Dianna Wheaton, a genetic counselor who previously directed the Southwest Eye Registry in Dallas, the FTD Disorders Registry will be a resource for ARFTL/LEFFTDS, GENFI, and biopharma companies conducting clinical trials. Importantly, it will remain independent of any given research network, trial, or company. Funded by FTD charities such as AFTD, the Bluefield Project, and the Tau Consortium, this registry will be a tax-exempt LLC designed in such a way that its participants own their data, Wheaton told the group.
People with any type of FTD, as well as their family members and caregivers, will be invited to join and enter data. This will be a contact registry that will do its own research via surveys and focus groups. It partnered with the Alzheimer’s Prevention Registry and its vendors to take advantage of the APR’s software design and marketing approaches, which have brought APR membership to 214,000 far. The partnership with the APR will allow the FTD Disorders Registry to learn from GeneMatch, a genotyping program the APR is piloting to help match people to therapeutic trials that require genetic information for recruitment. The FTD Disorders Registry will dip its toes into launch on May 13 at the AFTD Annual Conference in Minneapolis, offering access into the contact registry at that point; it will launch with full functions such as registration for research participation this June, Wheaton said.
ARTFL/LEFFTDS will also use resources already available through existing NINDS programs. One is the ongoing Parkinson’s Disease Biomarker Program. The PDBP already contains data and access to biosamples on 44 participants with PSP (see PDBP data), Sutherland told the audience. In addition, the PDBP features a data-management tool that the FTD Disorders Registry and ARTFL/LEFFTDS will use to create GUIDs, aka globally unique identifiers, for their participants. Like a social security number, a GUID enables tracking of people across studies or sites, such that an individual person’s clinical, genetic and other biological data can be linked. Biosamples collected under ARTFL/LEFFTDS are stored within NCRAD, the National Cell Repository for Alzheimer’s Disease. At present, a total of about 7,000 aliquots of CSF, blood, and RNA are at the cores and available for study, Sutherland said. More broadly, NINDS is building out other existing research resources to better accommodate FTD.
In its first year, ARTFL has enrolled 260 participants across the FTD spectrum, Boxer told the audience. For its part, LEFFTDS thus far has enrolled 39 people with a pathogenic tau mutation, 20 of them presymptomatic carriers, Brad Boeve of the Mayo Clinic, Rochester, told the audience. From across 13 pathogenic progranulin mutations, 55 people have signed up, 34 of them presymptomatic, and the group with C9ORF72 repeat expansion currently numbers 48 participants, 30 of them presymptomatic. Many more kindreds are known to scientists, though not all of them are published in the literature, Boeve noted. Some kindreds are quite large; for example, one carrying the N279K tau mutation numbers more than 320 members, of whom eight have joined the study, he said. Showing examples of large pedigrees, Boeve said that many families are unaware of the extent of the illness in their kindred, and that many individuals are not yet identified.

Carriers of pathogenic mutations located across the length of the progranulin gene have joined the LEFFTDs study of genetic FTD. [Courtesy of Brad Boeve, Mayo Clinic.]
The families who were previously known to the participating sites have become keenly interested in research since they learned about AFTFL/LEFFTDS, Boeve said. Additional families have come forward, too, and Boeve expects more people to make contact once the FTD Registry opens. He noted that the bottleneck in growing LEFFTDS are the centers, not the participants. “The families are coming. There are more interested individuals than there are staff to evaluate them all, but we aim to enroll and follow all who are interested,” Boeve said.
Estimating when a given LEFFTDS participant is likely to become symptomatic is difficult at present, Boeve acknowledged. While the age at which this happens is relatively tight within families of the same tau mutation, it can range from the 30s to the 70s within progranulin or C9 mutation families. For clinical trials to measure a cognitive or clinical outcome in these people, longitudinal studies need to identify biomarker changes that flag symptom onset and estimate how fast a patient is likely to progress. Mark Forman of Merck in Pennsylvania summed up the FTD Study Group’s consensus when he said, “The heterogeneity is the main challenge in how we can run trials, and biomarkers will be critical for their success.” For news on biomarkers in FTD, see Part 2 of this series.
To help with FTD trials, Albert Lo of Eli Lilly and Company in Rhode Island proposed building a shared infrastructure to support FTD therapy development. This platform, too, could draw inspiration from Alzheimer’s, where several initiatives are underway to streamline drug evaluation. One is Eisai’s Phase 2 trial of the BAN2401 anti-Aβ protofibril antibody. This trial is innovative because it tries to cut down both the length and needed number of patients with a Bayesian design that adapts randomization into dose groups based on frequent interim analyses. This trial design, the Phase 1 data on this antibody, and the composite outcome measure developed for it, were all recently published (Satlin et al., 2016; Logovinsky et al., 2016; Wang et al., 2016). Adaptive trial elements are a prominent feature of a large European initiative to develop a standing platform for Phase 1/2 Alzheimer’s prevention trials into which multiple pharma companies are to enter their investigational drugs (Ritchie et al., 2016). And of course, the DIAN-TU pioneered the idea of a trials platform built on an observational cohort by forming a pharma consortium in 2011. DIAN-TU’s ongoing Phase 2/3 trial comparing both solanezumab and gantenerumab to a shared placebo successfully completed enrollment last December (Dec 2011 conference news; Apr 2015 conference news; DIAN-TU press release).
How about it for FTD? At least in initial public comments at this early stage FTSG meeting, Lo’s proposal drew a tepid response. Would pharma companies withhold their best investigational drugs from such a platform? That has not been the case in DIAN-TU. Would management demur? The reticence in the room prompted a plea from Howard Feldman, University of California, San Diego. “Let’s remember there’s considerable urgency to this problem, and our progress is very slow. Unless we come together around something innovative, like this adaptive trial platform, we run the risk of continued very slow progress. It may not be for every company. Maybe it is for smaller, hungrier biotechs,” Feldman said.—Gabrielle Strobel
References
News Citations
- Regulators Tell Frontotemporal Dementia Community: We Play on Your Team
- AD-Related Dementias Summit 2016: Progress, Aims, Dollars
- New Ubiquitinated Inclusion Body Protein Identified
- Rademakers and Traynor Share Potamkin Prize
- First Data from GENFI1: Brain’s Insula Region Shrinks A Decade Before FTD
- Meet the Artful Leftie: NIH Jump-Starts U.S.-Canadian FTLD Cohorts
- WANTED: Biomarkers for Drug Trials in Frontotemporal Dementia
- DIAN Forms Pharma Consortium, Submits Treatment Trial Grant
- As DIAN Plans Trial Number Two, the Goal Is to Go Big
Conference Citations
Mutations Citations
Paper Citations
- Ratti A, Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem. 2016 Aug;138 Suppl 1:95-111. Epub 2016 Jun 15 PubMed.
- Todd TW, Petrucelli L. Insights into the pathogenic mechanisms of Chromosome 9 open reading frame 72 (C9orf72) repeat expansions. J Neurochem. 2016 Aug;138 Suppl 1:145-62. Epub 2016 Jun 15 PubMed.
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, van Minkelen R, Rombouts SA, Cardoso MJ, Clegg S, Espak M, Mead S, Thomas DL, De Vita E, Masellis M, Black SE, Freedman M, Keren R, MacIntosh BJ, Rogaeva E, Tang-Wai D, Tartaglia MC, Laforce R Jr, Tagliavini F, Tiraboschi P, Redaelli V, Prioni S, Grisoli M, Borroni B, Padovani A, Galimberti D, Scarpini E, Arighi A, Fumagalli G, Rowe JB, Coyle-Gilchrist I, Graff C, Fallström M, Jelic V, Ståhlbom AK, Andersson C, Thonberg H, Lilius L, Frisoni GB, Pievani M, Bocchetta M, Benussi L, Ghidoni R, Finger E, Sorbi S, Nacmias B, Lombardi G, Polito C, Warren JD, Ourselin S, Fox NC, Rossor MN. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015 Mar;14(3):253-62. Epub 2015 Feb 4 PubMed.
- Satlin A, Wang J, Logovinsky V, Berry S, Swanson C, Dhadda S, Berry DA. Design of a Bayesian adaptive phase 2 proof-of-concept trial for BAN2401, a putative disease-modifying monoclonal antibody for the treatment of Alzheimer's disease. Alzheimers Dement (N Y). 2016 Jan;2(1):1-12. Epub 2016 Feb 4 PubMed.
- Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, Basun H, Lannfelt L. Safety and tolerability of BAN2401--a clinical study in Alzheimer's disease with a protofibril selective Aβ antibody. Alzheimers Res Ther. 2016 Apr 6;8(1):14. PubMed.
- Wang J, Logovinsky V, Hendrix SB, Stanworth SH, Perdomo C, Xu L, Dhadda S, Do I, Rabe M, Luthman J, Cummings J, Satlin A. ADCOMS: a composite clinical outcome for prodromal Alzheimer's disease trials. J Neurol Neurosurg Psychiatry. 2016 Sep;87(9):993-9. Epub 2016 Mar 23 PubMed.
- Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, European Prevention of Alzheimer's Dementia (EPAD) Consortium. Development of interventions for the secondary prevention of Alzheimer's dementia: the European Prevention of Alzheimer's Dementia (EPAD) project. Lancet Psychiatry. 2016 Feb;3(2):179-86. Epub 2015 Dec 10 PubMed.
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.