Disturbed Sleep Exerts Toll on Memory and Neurodegeneration
Quick Links
The indispensable role of a good night’s sleep in memory and brain health was the subject of several talks at the Society for Neuroscience annual meeting, held November 11–15 in Washington, D.C. How might sleep-driven memory processes falter with age and Alzheimer’s pathology? It appears that as the brain gets older, accumulating Aβ and tau desynchronize the coordinated network oscillations that convert short-term memories to long-term ones during sleep. Sleep loss, in turn, ramps up production of Aβ and phosphorylated tau and kills off neurons in deep brain structures, never to be replaced.
- Aging disrupts the neural activity that solidifies memories during sleep.
- Alzheimer’s pathology also alters memory-consolidating neuronal waves during sleep.
- Skimping on sleep kills neurons in mice and pumps up p-tau.
While You Were Sleeping
Non-rapid eye movement sleep makes up about 80 percent of our sleeping hours. During NREM sleep, a trio of neuronal oscillations coordinates to consolidate memories formed during the day (Diekelmann and Born, 2010). Two of these oscillations, called the sleep spindle and the slow cortical wave, occur near the surface of the brain and can be measured by electroencephalography (EEG). Sleep spindles, which originate in the thalamus then propagate through the cortex, oscillate at 10 to 15 Hz, and get their name from the characteristic spiky trace they leave on EEG charts (see image below). The slow waves of the cortex hum along at about 0.8 Hz. Scientists believe that when these both align with the hippocampal sharp wave, then the brain is poised to convert short-term memories to long-term ones.
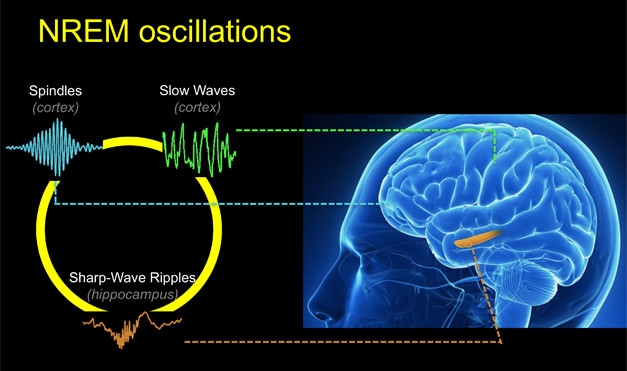
Synchronous Sleep. Coordination between three oscillations—hippocampal sharp-wave ripples, spindles, and slow waves—is crucial for consolidating memories while we sleep. [Courtesy of the Walker lab.]
Randolph Helfrich, working with Robert Knight and Matthew Walker at the University of California, Berkeley, wondered if the synchrony of the slow cortical wave and sleep spindle changes with age, and how that might affect memory. They recruited 20 participants around 20 years old and 32 older adults who averaged 74 years old. Each spent a night at the sleep lab hooked to an EEG monitor. Before they retired, Helfrich trained them on a word-pairing task. They learned to associate 120 real words with 120 nonsense ones. Helfrich tested subjects before they fell asleep and again after they woke up about eight hours later.
Helfrich’s findings were published in the December 14 Neuron (Helfrich et al., 2017). He found that the older people were less able to recall word pairs after a night’s rest. Their sleep oscillations were slightly off, too. While the spindle peaked just after the zenith of the slow cortical wave in the 20-year-olds, it crested about 125 ms earlier in the older adults (see image below). The more out of synch the two oscillations were, the worse the person performed on the memory task. Some of the younger volunteers did have premature spindles as well, but these seemed to affect memory performance only weakly. “Older adults express both waves, but they don’t come together as well in time,” said Helfrich. “If they don’t talk to one another, then there’s no reactivation of a short-term memory to put it in long-term storage.”
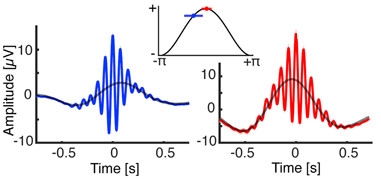
Twin Peaks.
In young adults (right) the sleep spindle (red) peaks slightly after the slow cortical wave (black curve). In older adults (left) the spindle (blue) peaks just before. [Neuron, Helfrich et al.]
Knowing that the brain shrinks with age, Helfrich wanted to test whether atrophy in any particular brain region predicted perturbed coordination of the two oscillations. The researchers analyzed magnetic resonance imaging (MRI) scans of each participant. They found that a smaller medial prefrontal cortex correlated with poor synchronization between spindles and slow waves. The medial prefrontal cortex plays an important role in the generation of slow cortical waves (Saletin et al., 2013).
In his paper, Helfrich and co-authors proposed that noninvasive stimulation of the brain during sleep might be able to restore the precise coordination between spindles and slow cortical waves to match that of young adults. Perhaps that would reduce memory decline in aging, they suggested.
Could Alzheimer’s pathology further skew this delicate synchrony? Walker’s group previously found that Aβ deposition in the cortex correlated with fewer slow cortical waves (Jun 2015 news). Joseph Winer, in the lab of Bill Jagust at UC Berkeley, wondered if tau accumulation in the medial temporal cortex might disrupt the sleep spindles that originate close by in the thalamus.
To test this hypothesis, Winer recruited 23 cognitively normal subjects from the Berkeley Aging Cohort Study who had had an amyloid and a tau PET scan. Their average age was 76. Each had an overnight sleep study that measured sleep spindles and slow cortical waves by EEG.
As previously reported by Walker, Aβ deposition in the cortex correlated with disrupted slow cortical waves. Wave amplitudes were smaller in many areas where plaques accumulated. The strongest association was in the prefrontal cortex, where slow waves originate; the more plaques there, the lower the wave amplitude. On the other hand, tau accumulation in the hippocampus decreased the frequency of sleep spindles. This correlation was strongest in parietal areas, where spindle frequencies tend to hover around the top of the 10–15 Hz range; the more tau there, the lower the spindle frequency.
Further, tau deposition correlated with poor synchrony between slow cortical waves and sleep spindles. “These findings give us a window into how [AD] pathology in healthy people disrupts important brain processes during sleep,” Winer told Alzforum.
Jagust agreed. “We need to study more subjects to be certain, but I think our data points to the idea that at least some of the changes in coupling might be related to the deposition of tau,” he wrote to Alzforum.
Researchers at SfN asked if people with Aβ or tau pathology report sleep problems. Winer replied that only amyloid deposition correlates with complaints on sleep questionnaires. The relationship is likely bi-directional, he said, because sleep helps clear waste from the brain, and Aβ pathology in turn impairs sleep (May 2014 conference news). However, it remains to be seen whether better sleep will improve memory once Aβ and tau have already deposited, Winer added. The team next plans to examine whether Aβ/tau-related sleep disturbances correlate with worse memory.
While You Were Not Sleeping
Could pulling a few all-nighters per week to binge-watch the latest hit show permanently damage the brain? Possibly, according to preliminary research by Sigrid Veasey at the Perelman School of Medicine, University of Pennsylvania, Philadelphia. Her work suggests that temporarily depriving mice of their normal sleep accelerates tau phosphorylation in the locus coeruleus, which leads to rampant neurodegeneration there. Nestled in the brainstem, the locus coeruleus is active when people are awake, and it projects all over the brain. It has been proposed as a start site for AD pathology (Elobeid et al., 2012).
Veasey previously found that chronic sleep loss in wild-type mice led LC neurons to produce more Aβ, which in turn prompted tau hyperphosphorylation (unpublished data). She reported that sleep loss leads to a drastic loss of neurons in the LC (Zhang et al., 2014). In the current study, she tested whether tau contributed to LC neuron loss, and whether those neurons were replaced.
The troubling answers appear to be yes and no. Veasey and colleagues subjected eight-week-old mice to one month of chronic sleep loss. For three consecutive days each week, tempting new climbing toys encouraged mice to play instead of rest during eight of their normal 12 daytime sleep hours. While they were allowed to rest and catch up on sleep for the remainder of the 24-hour period, they only made up about half of what they had lost.
Four weeks after intervention ended, Veasey found about 30 percent fewer neurons in the LC of sleep-deprived mice than in the LC of mice that slept soundly. Meanwhile, there were four times more activated astrocytes and twice as many activated microglia in the LC of sleep-deprived than control mice. In addition, p-tau, as detected with the AT8 antibody, turned up in neurons and in microglia.
Nine months after this one month of sleep loss, the mice had trouble remembering as judged by an object placement task. This test measures how long mice spend exploring each of two familiar objects, one having been moved to a new location. In addition, 30 percent of neurons in the amygdala had died and p-tau had not gone away, but had instead spread to the hippocampus. Repeating the experiment with tau knockouts, or in mice injected with tau siRNA just before sleep deprivation, completely protected against neuron loss.
While normal tau mediated injury from sleep loss, mutant tau was even worse. P301S tau mice that were sleep-deprived lost twice as many LC neurons as did wild-type animals put through the same wringer. Again, these neurons were not replaced nine months later. “Sleep loss can irreversibly injure the brain,” Veasey said. “This is the first work to show that conclusively.” This neuronal loss came with massive increases in p-tau and tau aggregates labeled by AT8 and MC-1, respectively, in both the LC and entorhinal cortex.
To rule out overstimulation of mice as the cause of this damage, as opposed to lack of sleep, Veasey’s group is looking at a second type of deprivation, namely fragmented sleep, seen in mice housed in cages that gently nudge the animals awake every minute. In those mice, too, the researchers are seeing more p-tau and neuron loss in the LC.
Audience members clamored to know if there was a way to reverse the impact of temporary sleep loss. Veasey emphasized that the p-tau remained nine months after the treatment and the LC neurons never came back. She believes the changes are permanent, but there may be some compensation for the lost neurons. Eckhard Mandelkow of the DZNE in Bonn, Germany, thought that likely. While he was shocked by the amount of neuron loss, he cautioned about overinterpreting the tau data, pointing out that p-tau is not the same as tangles and that the MCI antibody is not specific for tangles either.
Still, in keeping with Winer’s data, Veasey thinks there may be a vicious cycle where sleep loss causes tau abnormalities, which in turn cause more sleep problems. One person in the audience noted that people with Down’s syndrome, who are at extremely high risk for early onset AD, accumulate a lot of tau in their LC in their teenage years, and they also suffer from disturbed sleep.—Gwyneth Dickey Zakaib
References
News Citations
- Does Amyloid Disturb the Slow Waves of Slumber—and Memory?
- Glymphatic Flow, Sleep, microRNA Are Frontiers in Alzheimer’s Research
Paper Citations
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010 Feb;11(2):114-26. Epub 2010 Jan 4 PubMed.
- Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP. Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron. 2017 Dec 12; PubMed.
- Saletin JM, van der Helm E, Walker MP. Structural brain correlates of human sleep oscillations. Neuroimage. 2013 Dec;83:658-68. Epub 2013 Jun 14 PubMed.
- Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012 Jan;123(1):97-104. PubMed.
- Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, Wang MM, Reid S, Lai D, Davis JG, Baur JA, Veasey S. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci. 2014 Mar 19;34(12):4418-31. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.