Unbiased Screen Fingers TREM2 Ligands That Promote Aβ Uptake
Quick Links
Since scientists found that variants in the gene for TREM2 triple a person’s risk for Alzheimer’s, they have wondered what the protein does in health and disease. Talks and posters at “Microglia in the Brain,” the Keystone symposium that ran June 12-16 in Colorado, addressed some of the main issues, including what ligands bind the cell surface receptor and whether it helps or hinders Aβ pathology in mouse models of AD.
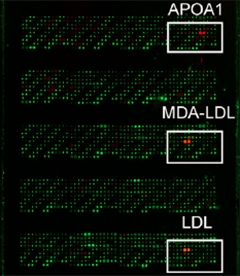
Binding Array.
From a large library of extracellular and membrane proteins, ApoA1, LDL, and malondialdehyde-modified LDL bound TREM2. [Image courtesy of Felix Yeh and Neuron.]
The TREM2 gene codes for a single transmembrane protein that partners with the adapter protein DAP12. Scientists believe that the two proteins trigger a series of downstream signaling events, but what kicks them into gear remains a bit of a mystery since few ligands for TREM2 have been identified. Felix Yeh, working with Morgan Sheng, Lino Gonzalez, and colleagues at Genentech, South San Francisco, decided to tackle this issue by using an unbiased screen. Yeh used a protein microarray developed at Genentech to study the interactions of extracellular proteins. The array contains thousands of secreted and transmembrane proteins that can be tested against proteins of choice. When Yeh probed it with the extracellular domain of TREM2, it bound several lipoproteins, including apolipoprotein A1 (ApoA1), low-density lipoprotein (LDL), and unc-5 homology B (see image at left). Using a biophysical technique called bio-layer interferometry, which directly measures protein-protein interactions, he confirmed that LDL and ApoA1 bind TREM2. UNC5B failed to bind in this assay.
This made Yeh wonder if TREM2 might bind apolipoproteins, including ApoE and ApoJ, aka clusterin, that can alter the risk for AD. Sure enough, in the interferometry assay, he found that both, when in lipidated form, bound the receptor. Yeh said his initial screen likely missed these proteins because they are not lipidated on the microarray.
Does cellular TREM2 bind these proteins? Yeh and colleagues tested this in HEK293 transfected with a doxycycline-driven TREM2 gene. When he induced expression of the surface receptor, the cells took up acetylated LDL (acetylation of the protein prevents its binding to the LDL receptor, which is also expressed on HEK cells and might interfere with the uptake assay). The TREM2-expressing HEK cells also took up lipidated ApoE and lipidated clusterin. Turning to microglia, the researchers found that cells from TREM2 knockout mice took up LDL much more slowly than did cells from wild-type mice, supporting the idea that TREM2 plays a role in microglial lipoprotein uptake.

Lipoprotein Uptake.
HEK293 cells expressing wild-type TREM2 (top) readily ingest LDL (right); cells expressing Y38C (middle) and R47H (bottom) variants of TREM2 do not. [Image courtesy of Felix Yeh and Neuron.]
To relate these observations to disease, the researchers tested a variety of TREM2 mutations in the protein-binding and cell-uptake assays. They found that the Y38C and T66M mutations, which cause Nasu-Hakola disease and have been linked to frontotemporal dementia, abolished TREM2 binding to LDL, clusterin, and ApoE. The AD risk alleles R62H, D87N, and R47H, in that order, progressively weakened binding, such that R47H TREM2 bound about half as much lipoprotein as did wild-type receptor. Uptake assays told a slightly different story, perhaps because of the presence of other receptors in the cells and different sensitivities of the assays, said Yeh. HEK cells expressing Y38C and T66M TREM2 took up about a fifth as much acetylated LDL (see image at right), which gibes with their reduced binding. Even though R47H TREM2 binds more tightly to the lipoproteins, cells expressing this mutant took up similar amounts of LDL to the Y38C and T66M cells. R47H cells did take up about half as much clusterin as did wild-type cells, though. Finally, cells expressing the R62H and D87N variants, which have the highest affinity for lipoprotein among the tested mutants, took up nearly normal amounts of clusterin but slightly less LDL.
Lastly, Yeh tested if these perturbations in binding and uptake of apolipoproteins weakened microglial processing of Aβ. He reported that wild-type, TREM2+/- heterozygotes, and TREM2 knockout cells all took up very little free Aβ. In contrast, all cells imbibed vastly more Aβ when it came combined with LDL or clusterin. However, there was a marked TREM2 dose-dependency—homozygotes took up about twice as much as did knockouts, with the heterozygotes falling in between. Since Aβ binds ApoE and clusterin, and both proteins are found not only in the extracellular space but in amyloid plaques, these findings raise the possibility that microglia are particularly suited to digesting plaque-associated Aβ, suggested Yeh.
Researchers at Keystone were impressed by the findings, which were published in the July 20 Neuron, and Yeh’s poster drew a large crowd. “There have been some attempts to find TREM2 ligands, but this stands out for the rigor of the analysis,” said Gary Landreth, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis. Using a candidate approach others had found that ApoE binds TREM2 and that the R47H mutation weakens the interaction, but they had not made the connection to Aβ uptake (see Atagi et al., 2015; Bailey et al., 2015).
Yeh’s findings may be directly relevant to human biology. He said that among blood samples donated by 1,500 volunteers, he found one R62H carrier whose macrophages took up less lipoprotein-Aβ than did macrophages isolated from an age-, sex-, and ethnicity-matched control.
What about other microglial responses that TREM2 mutations might perturb? In her poster, Fargol Mazaheri, who works in Christian Haass’ lab at the German Center for Neurodegenerative Diseases, Munich, described a transcriptomics approach to nail down what knocking out TREM2 does to a cell. She isolated microglia from normal mice and from TREM2 knockouts with a fluorescent-activated cell sorter. Then she quantified transcripts using nanostring technology, à la Oleg Butovsky’s seminal study (see Butovsky et al., 2014).
Mazaheri’s data suggest that TREM2 functions in microglial homeostasis, migration, and inflammatory responses. Among 122 genes thought important for homeostasis, many were upregulated in TREM2 KOs, while genes involved in inflammation and migration were turned down. Mazaheri tested cell motility by juxtaposing old and young organotypic brain tissue slices together in culture. In this scenario, CD68-positive cells (microglia, macrophages, and monocytes) usually migrate from the young tissue toward the old, she explained to Alzforum, perhaps to scavenge dead or dying cells. Not in the case of TREM2 KO tissue. To test mobility specifically in microglia, Mazaheri took advantage of CRISPR technology to knock TREM2 out of N9 microglial cells. This repressed mobility-related genes and chemotaxis. In preliminary experiments she tested microglial migration in vivo in mice using a laser injury model, in which microglia flock to the site of a small wound burned into tissue. Mazaheri found that TREM2 knockout microglial are sluggish in this assay. The findings dovetail with reports that in TREM2-deficient AD models, microglia don’t surround plaques as readily as they normally do (see Jun 2014 news and May 2016 news).
Researchers at the labs of Landreth and Bruce Lamb, now also at Stark, have also used CRISPR/CAS9 to study TREM2 biology. They generated R47H TREM2 heterozygotes and crossed them to APPPS1 mice. Preliminary data point to fewer microglia and reduced microglial proliferation in these crosses. The R47H TREM2/APPPS1 mice also have fewer plaques at four months of age than APP controls. This fits with prior reports from these labs that APP/PS1 mice lacking TREM2 had fewer plaques in the hippocampus than controls (see Dec 2014 conference news and Jay et al., 2015).
Those earlier reports surprised the field, which had come to expect that TREM2 might play a role in microglial plaque clearance. The data also contrasted with results from Marco Colonna at Washington University, St. Louis, who found more hippocampal plaques in 5xFAD mice lacking TREM2 (see Feb 2015 news and Feb 2015 conference coverage). Researchers were left wondering whether TREM2 promotes clearance or accumulation of plaques.
In his talk, Landreth seemed to settle this controversy. Updating his characterization of the APP/PS1 TREM2 knockouts, he said his lab’s data now better matches that of WashU group. “We are now on the same page with Colonna and colleagues,” he told Alzforum. The reason? Aging. Taylor Jay, a graduate student working for both Landreth and Lamb, had initially necropsied four-month-old mice, but when she examined eight-month-old animals, she saw that the TREM2 knockouts had accumulated more plaques in the hippocampus than the regular APP/PS1 mice, just as Colonna’s group had seen in their eight-month-old mice. “We see a distinct progression-dependent change in plaque burden” said Landreth. Researchers at Keystone wondered what might underlie that change, but Landreth was not sure. He suspects a dynamic up-down regulation of plaque accumulation related to TREM2 function, but what drives that dynamic is unclear, he said.
Further analysis will tell if plaque burden changes as the R47H TREM2/APPPS1 animals age as well.—Tom Fagan
References
News Citations
- TREM2 Mystery: Altered Microglia, No Effect on Plaques
- Barrier Function: TREM2 Helps Microglia to Compact Amyloid Plaques
- TREM2 Data Surprise at SfN Annual Meeting
- TREM2 Buoys Microglial Disaster Relief Efforts in AD and Stroke
- United in Confusion: TREM2 Puzzles Researchers in Taos
Research Models Citations
Paper Citations
- Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J Biol Chem. 2015 Oct 23;290(43):26043-50. Epub 2015 Sep 15 PubMed.
- Bailey CC, DeVaux LB, Farzan M. The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J Biol Chem. 2015 Oct 23;290(43):26033-42. Epub 2015 Sep 15 PubMed.
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014 Jan;17(1):131-43. Epub 2013 Dec 8 PubMed.
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015 Mar 9;212(3):287-95. Epub 2015 Mar 2 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.