Stressed Out: RNA-binding Protein Inhabits Granules in ALS, FTD
Quick Links
Panning the cerebrospinal fluid of people with amyotrophic lateral sclerosis, researchers discovered a new pathological marker in RNA-binding motif 45 (RBM45). In diseased neurons, this protein hangs out in cytoplasmic stress granules, a familiar compartment for scientists interested in ALS. It also occupies nuclear stress bodies, a new structure for the field. “How the nucleus responds to stress has been unexplored territory for ALS and other neurodegenerative disease,” said Robert Bowser of the Barrow Neurological Institute in Phoenix, who presented the work at RNA Metabolism in Neurological Disease, a Society for Neuroscience satellite symposium held October 15-16 in Chicago.

RBM45 Domains.
[Courtesy of Li et al., Scientific Reports.]
Like the well-known ALS proteins TDP-43 and FUS, RBM45 contains RNA-binding domains. While it lacks the prion-like low-complexity sequences of the other two proteins, it sports a nuclear localization sequence, as FUS does (see image above). Though no current evidence indicates genetic variants near the RBM45 gene associate with ALS or any other neurodegenerative disease, Bowser and collaborators are checking sequences of people with familial ALS.
Scientists know little about RBM45. It is expressed highly in the brain and upregulated following spinal-cord injury (Mladinic et al., 2010). Bowser and colleagues observed that while the protein appears to be predominantly nuclear, some collects in the cytoplasm. In both cellular locations, it makes a speckled pattern consistent with the large protein/RNA complexes formed by RNA-binding proteins. Bowser suspects it may shuttle between the two compartments. RBM45 also condenses into cytoplasmic inclusions in the motor neurons of ALS patients. There, it co-localizes with TDP-43 and ubiquitin, common pathological markers of the disease. It also forms cytoplasmic inclusions in people who died of frontotemporal lobar degeneration or Alzheimer’s disease (Collins et al., 2012).
A New, Nuclear Stage for Pathology
Bowser reported in Chicago that the RBM45 also formed nuclear inclusions. These were much smaller than typical cytoplasmic stress granules, Bowser said, and did not co-localize with TDP-43 or ubiquitin, common markers for pathological aggregates in the cytoplasm. They formed in both neurons and glia. In autopsy samples of hippocampi and spinal cords, the average nucleus contained about three of these puncta. In ALS, FTD, and AD cases, there were about 10.
What were these inclusions? Testing both neural and non-neuronal cell lines with antibodies to various nuclear substructures, Bowser discovered the puncta contained the heat shock transcription factor 1 (HSF1). In addition to activating genes in response to high temperatures, HSF1 helps generate nuclear stress bodies. Very little is known about these structures, but they are believed to protect against stress by recruiting transcription and splicing factors to DNA to modulate gene expression, said Bowser. Notably, nuclear stress bodies only occur in primate cells, he said.
To see if nuclear stress bodies are important in disease states, the researchers re-examined the autopsy tissue. They found more puncta containing scaffold attachment factor B (SAFB), another stress body marker, in the ALS and FTD cases than in the controls. Now, the scientists are working to understand the function of nuclear stress bodies and how they act on gene expression in chronically stressed neurons during neurodegeneration, Bowser told Alzforum.
Plenty of Action in the Cytoplasm
Even as they analyze the RBM45-containing stress bodies, Bowser’s group is working to understand the protein and its interactions at a molecular level. In a poster, Yang Li reported that individual RBM45 proteins associate in multimers. Li got hints of this first from co-immunoprecipitation experiments and then by using a chemical cross-linker to stabilize multimers. In the presence of the cross-linker, RBM45 migration in polyacrylamide gels corresponded to that of a pentamer. RBM45 might form a ring, Li speculated, and two rings might stack to form a decamer. Using a series of deletion mutants, she determined that the mid-section of the protein, which she named the homo-oligomer assembly (HOA) domain, was necessary for this oligomerization (see image above).
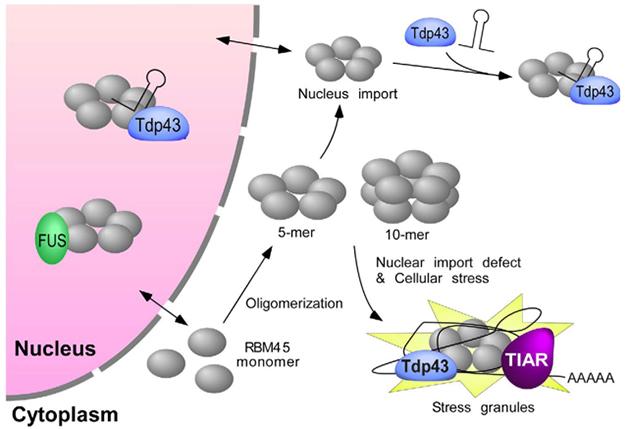
RBM45 interactors. RBM45 monomers assemble into multimers that associate with TDP-43 via a bridging RNA. Multimers may end up in stress granules in the cytoplasm, or stress bodies in the nucleus. [Courtesy of Li et al., Scientific Reports.]
What about interactions with other proteins? Li has not yet examined nuclear stress bodies but did study how RBM45 interacts with TDP-43 in the cytoplasm. The two proteins co-immunoprecipitated—but not, however, if Li treated the cells with RNase. She thinks they may bind and co-regulate the same transcripts. Because RBM45 also needed the HOA to associate with TDP-43, Li thinks RBM45 must bind RNAs as an oligomer. The researchers are now trying to identify the RNAs that bind both RBM43 and TDP-43 (Li et al., 2015).
In another recent paper, Bowser and colleagues focused on the cytoplasmic activities of RBM45. They found that like TDP-43 and FUS, it moved from the nucleus to the cytoplasm in cultured motor neurons that were undergoing oxidative stress. There, it sometimes co-localized with stress granule markers, but it also bound KEAP1, a suppressor of the cell’s oxidative stress response. Mislocalized, cytoplasmic RBM45 prevented KEAP1 degradation, diminishing the stress response and leaving neurons vulnerable to attack by free radicals. During ALS, motor neurons experience oxidative stress, so RBM45’s actions could be a reason they fail to protect themselves (Bakkar et al., 2015).
In sum, RBM45 associates with stress-induced structures both in the cytoplasm and in the nucleus of neurons afflicted with neurodegenerative disease, suggesting the nucleus may be a new locus of pathology. “It is another protein that probably plays roles in multiple steps of RNA metabolism and is altered during times of stress and injury,” Bowser told Alzforum. “It opens up new opportunities to explore how nuclear stress is critical for determining long-term cell survival of neurons.”
Peter Ash of Boston University, who did not participate in the studies, said more work is needed to understand the implications of RBM45 in nuclear stress structures. “Perhaps RBM45 is regulating a number of important stress-responsive pathways, such as KEAP1 and HSF1,” he speculated. “Perhaps RBM45 function is key to the sort of appropriate and transient stress response that is lost in ALS.”
Paul Taylor of St. Jude Children’s Research Hospital in Memphis, Tennessee, said the work was important. “There may be a direct link between RBM45 function and disease, although this remains to be firmly established,” commented Taylor, who co-organized the symposium. “The biology of this protein is a strong reminder that the pathobiology of ALS likely involves disturbance of multiple RNA-protein assemblies that reside in both nuclear and cytosolic compartments.”
Amelie Gubitz of the National Institute of Neurological Disorders and Stroke in Bethesda, Maryland, praised the insights derived from a fishing expedition in CSF. “I think this type of patient biosample-based research has great potential in terms of developing ALS biomarkers and obtaining new insights into the molecular mechanisms of the disease,” she told Alzforum. Bowser said he is interested in exploring RBM45’s potential as a biomarker, but has not yet checked how its levels correlate with disease onset or progression.—Amber Dance
References
Paper Citations
- Mladinic M, Lefèvre C, Del Bel E, Nicholls J, Digby M. Developmental changes of gene expression after spinal cord injury in neonatal opossums. Brain Res. 2010 Dec 2;1363:20-39. Epub 2010 Sep 16 PubMed.
- Collins M, Riascos D, Kovalik T, An J, Krupa K, Hood BL, Conrads TP, Renton AE, Traynor BJ, Bowser R. The RNA-binding motif 45 (RBM45) protein accumulates in inclusion bodies in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) patients. Acta Neuropathol. 2012 Nov;124(5):717-32. PubMed.
- Li Y, Collins M, Geiser R, Bakkar N, Riascos D, Bowser R. RBM45 homo-oligomerization mediates association with ALS-linked proteins and stress granules. Sci Rep. 2015 Sep 22;5:14262. PubMed.
- Bakkar N, Kousari A, Kovalik T, Li Y, Bowser R. RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1. Mol Cell Biol. 2015 Jul;35(14):2385-99. Epub 2015 May 4 PubMed.
Further Reading
Papers
- Borza LR. A review on the cause-effect relationship between oxidative stress and toxic proteins in the pathogenesis of neurodegenerative diseases. Rev Med Chir Soc Med Nat Iasi. 2014 Jan-Mar;118(1):19-27. PubMed.
- Tao Z, Wang H, Xia Q, Li K, Li K, Jiang X, Xu G, Wang G, Ying Z. Nucleolar stress and impaired stress granule formation contribute to C9orf72 RAN translation-induced cytotoxicity. Hum Mol Genet. 2015 May 1;24(9):2426-41. Epub 2015 Jan 9 PubMed.
- Ash PE, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B. Pathological stress granules in Alzheimer's disease. Brain Res. 2014 Oct 10;1584:52-8. Epub 2014 Aug 7 PubMed.
- Boyd JD, Lee-Armandt JP, Feiler MS, Zauur N, Liu M, Kraemer B, Concannon JB, Ebata A, Wolozin B, Glicksman MA. A High-Content Screen Identifies Novel Compounds That Inhibit Stress-Induced TDP-43 Cellular Aggregation and Associated Cytotoxicity. J Biomol Screen. 2013 Sep 9; PubMed.
- Vanderweyde T, Youmans K, Liu-Yesucevitz L, Wolozin B. Role of Stress Granules and RNA-Binding Proteins in Neurodegeneration: A Mini-Review. Gerontology. 2013;59(6):524-33. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.