TGF-β Gives TLC to Newborn Neurons
Quick Links
Transforming growth factor-β (TGF-β), a cytokine that regulates cell growth and differentiation during development, appears to nurture adult neurogenesis. When scientists led by Tony Wyss-Coray, Stanford School of Medicine, California, dampened the TGF-β signaling pathway in adult mice, fewer newborn neurons matured or integrated into new circuits. Revving up the pathway had the opposite effect. The paper identifies the receptor through which TGF-β signals—ALK5. Curiously, this also appears to be the receptor for the recently reported rejuvenating peripheral growth factor GDF11. The novel mechanism may point to targets for improving learning and memory, according to the May 25 Nature Neuroscience paper.
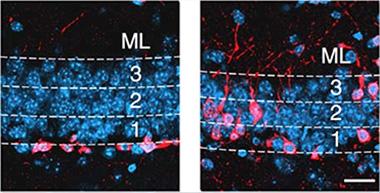
Newborn neurons (pink) from mice overexpressing ALK5 (right) migrated farther into the granular cell layer than those from control mice (left). [Image courtesy of Nature Neuroscience, He et al., 2014.]
“We’ve suspected for some time that TGF-β is involved in adult neurogenesis, but no one had done the experiment to demonstrate it in an elegant way,” commented Terrence Town, University of Southern California, Los Angeles.
Wyss-Coray's group had previously reported that TGF-β was highly expressed in the subgranular zone of the dentate gyrus, a neurogenic hotspot, hinting that the protein played a role in forming new neurons (see Luo et al., 2006). However, the researchers found that widespread overproduction of TGF-β by astrocytes suppressed the proliferation of progenitor cells in mice (see Buckwalter et al., 2006). First author Yingbo He and colleagues then wondered if TGF-β benefitted newborn neurons directly. To examine this, they looked in adult mouse brains to see where a downstream gene product of TGF-β signaling—p-Smad2—appeared most prominently. Sure enough, it was highest in postmitotic immature neurons and recently generated mature neurons of the dentate gyrus.
To see if TGF-β had an impact on neurogenesis, He and colleagues targeted its ALK5 receptor. They ablated it genetically or injected shRNA into the dentate gyrus. As a result, fewer newborn neurons survived and rather than migrating to the molecular layer of the dentate gyrus, they languished in the granular cell layer, where they grew stunted dendrites or none at all. When the researchers activated ALK5 in the forebrain, more newborn neurons survived. They migrated farther into the granule cell layer (see image above) and developed a higher number of complex dendrites than wild-type cells. “We were stunned to see how dramatically different neurogenesis looked if we activated TGF-β or inhibited it,” Wyss-Coray told Alzforum. In an email to Alzforum, Li-Huei Tsai, Massachusetts Institute of Technology, Cambridge, commended the group for demonstrating the consequences of both gain and loss of function of ALK5.
Compared with controls, mice with enhanced ALK5 signaling preferred to explore novel environments in a Y-maze, and froze more in a contextual fear-conditioning task, suggesting better working and spatial memory.
Town found the behavioral data striking, saying it strengthens the notion that more adult neurogenesis benefits memory. “That makes things relevant for Alzheimer's disease,” he said. “If these data hold up and we can promote neurogenesis in the context of AD, it may ultimately be beneficial.”
Wyss-Coray said he plans to see whether amping up this signaling pathway improves memory in aged animals or models of Alzheimer’s. He reported previously that knocking the pathway down accelerates pathology and neurodegeneration in AD mouse models (see Tesseur et al., 2006). His group has also been looking for small molecules that enhance TGF-β activity. Town pointed out that a targeted delivery system would be ideal to modify only select populations of cells in the dentate gyrus, because TGF-β influences various cell types in different ways. For instance, Town had previously found that blocking TGF-β signaling in peripheral macrophages stimulated them to cross the blood-brain barrier and clear amyloid deposits (see Jun 2008 news story).
The authors note that other ligands could be responsible for the positive effects of ALK5 signaling. Members of the growth and differentiation factor (GDF) family also activate this receptor. “It would be important to dissect out the effects of the different ligands of the ALK5 signaling pathway, as well as its downstream mediators,” said Orly Lazarov, University of Illinois at Chicago. “This paper is a good beginning of a finely detailed analysis of this pathway.” Interestingly, scientists recently found that GDF11 circulating in the blood of young mice rejuvenated heart and skeletal muscle, revitalized brain capillaries, and boosted neurogenesis in older animals (see May 2014 conference story). Some of the benefits of GDF11 could be due to an impact on neurogenesis, said Wyss-Coray.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Luo J, Lin AH, Masliah E, Wyss-Coray T. Bioluminescence imaging of Smad signaling in living mice shows correlation with excitotoxic neurodegeneration. Proc Natl Acad Sci U S A. 2006 Nov 28;103(48):18326-31. PubMed. Correction.
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006 Jul;169(1):154-64. PubMed.
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006 Nov;116(11):3060-9. PubMed.
External Citations
Further Reading
Papers
- Roussa E, von Bohlen und Halbach O, Krieglstein K. TGF-beta in dopamine neuron development, maintenance and neuroprotection. Adv Exp Med Biol. 2009;651:81-90. PubMed.
- Míguez DG, Gil-Guiñón E, Pons S, Martí E. Smad2 and Smad3 cooperate and antagonize simultaneously in vertebrate neurogenesis. J Cell Sci. 2013 Dec 1;126(Pt 23):5335-43. Epub 2013 Oct 8 PubMed.
- Tapia-González S, Muñoz MD, Cuartero MI, Sánchez-Capelo A. Smad3 is required for the survival of proliferative intermediate progenitor cells in the dentate gyrus of adult mice. Cell Commun Signal. 2013 Dec 13;11:93. PubMed.
- Williams G, Zentar MP, Gajendra S, Sonego M, Doherty P, Lalli G. Transcriptional basis for the inhibition of neural stem cell proliferation and migration by the TGFβ-family member GDF11. PLoS One. 2013;8(11):e78478. Epub 2013 Nov 7 PubMed.
- Marschallinger J, Krampert M, Couillard-Despres S, Heuchel R, Bogdahn U, Aigner L. Age-dependent and differential effects of Smad7ΔEx1 on neural progenitor cell proliferation and on neurogenesis. Exp Gerontol. 2014 May 24;57C:149-154. PubMed.
Primary Papers
- He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, Fainberg N, Wyss-Coray T. ALK5-dependent TGF-β signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci. 2014 Jul;17(7):943-52. Epub 2014 May 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.