Traumatic Tau: Filaments from CTE Share Distinct Structure
Quick Links
Whether sustained on the field or in the ring, repeated blows to the head result in a distinctive strain of tau filaments in the brain. In the March 20 Nature, researchers led by Sjors Scheres and Michel Goedert of the MRC Laboratory of Molecular Biology in Cambridge, U.K., unveiled the structure of tau filaments from the brains of three people—one former American football player and two former boxers—who died with chronic traumatic encephalopathy (CTE). Similar to previously reported structures from people with Alzheimer’s disease, the CTE filaments comprised rungs of coupled C-shaped protofilaments. However, the CTE Cs had wider openings, and cradled a mysterious molecule that the researchers proposed could hold clues about CTE-tau’s traumatic origins, and perhaps even point to therapeutic strategies.
- Tau filaments from CTE shared common structure.
- Similar to AD: C-shaped protofilaments made of R3 and R4 domains.
- Different from AD: Mystery molecule nestled in fold.
“Head trauma is about the most nonspecific thing you can imagine, and yet, it leads to this very specific structure,” Scheres marveled to Alzforum.
“This is yet another very important study to show that tau lesions are different among tauopathies,” commented Mathias Jucker of the German Center for Neurodegenerative Diseases in Tübingen.
First described in former boxers as “punch-drunk syndrome,” CTE is a neurodegenerative tauopathy that is now known to affect retired players of other contact sports, retired soldiers, and people who suffered repeated physical abuse. Upon autopsy, the brains of people with CTE have deposits of hyperphosphorylated tau in neurons, astrocytes, and cell processes around blood vessels—a distribution pattern distinct from that observed in people with AD. Tau filaments in CTE and AD also have a major commonality in that both contain 3R- and 4R-tau isoforms. Four-R-tau harbors four microtubule binding domains—dubbed R1-R4—while 3R-tau is missing the second one, R2.
In 2017, Goedert and Scheres used cryo-electron microscopy to resolve the structure of AD-tau filaments, reporting that they incorporated only the common R3 and R4 domains into their C-shaped protofilament cores (Jul 2017 news). In 2018, they described a wildly different, J-shaped structure of tau from Pick’s disease, a pure 3R tauopathy (Aug 2018 news).
How would CTE-tau compare? To find out, the researchers collaborated with Kathy Newell of the University of Kansas School of Medicine in Kansas City and Bernardino Ghetti at the Indiana University School of Medicine in Indianapolis, who diagnosed the three former athletes with CTE via post-mortem neuropathological examination and provided tissue samples. First author Benjamin Falcon and colleagues then set out to resolve the tau filaments extracted from their brains with cryoEM. Prior to taking the freezing plunge, the brain samples were probed by various staining and labeling methods. In all three cases, negative-stain electron microscopy revealed a predominant helical tau filament, dubbed Type I, which accounted for 90 percent of the tau filaments. Type I CTE filaments were distinctive from the paired helical filaments (PHFs) and straight filaments identified in AD, the narrow and wide filaments in Pick’s disease, and recombinant filaments assembled in the presence of heparin. The remaining 10 percent of filaments, dubbed Type II, had a similar shape to PHFs, with regular bulges along the helical axis (see below). Immunoblotting confirmed that both types of CTE tau filament contained all six isoforms of tau. Immunogold electron microscopy indicated that the R3 and R4 domains made up the structured core, while the R1 and R2 domains formed part of an unstructured fuzzy coat.
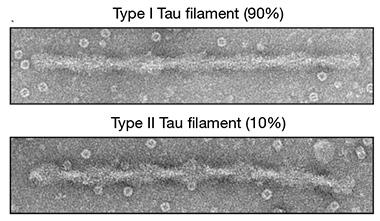
Filament Flavors. Negative-stain electron microscopy revealed two helical types of tau filaments in people with CTE. Type II filaments resembled paired helical filaments (PHFs) identified in AD. [Courtesy of Falcon et al., Nature, 2019.]
Using cryoEM and sophisticated software, the researchers resolved the Type I CTE filaments to an unprecedented 2.3Å, the highest resolution ever reported for an amyloid structure. Reminiscent of AD-tau, the CTE-tau filaments comprised stacked protofilaments made up of back-to-back, two-ply C-shaped structures, each made up of a strip of eight β-strands folded in half by a triangular β-helical turn.
However, notable differences also emerged. For one, CTE tau had a more open C-shape than AD tau, and a longer open space within its β-helical fold (see image below). Most strikingly, the roomier β-helical fold of CTE tau cradled a distinctive extra density (see purple dot below).

Mystery Molecule. Compared with the AD fold (middle), the CTE fold had a more open C-shape, and contained an extra density (purple dot). Overlay, right. [Courtesy of Falcon et al., Nature, 2019.]
Separately, the researchers generated a 3.4Å resolution structure of Type II filaments, and found that while the protofilaments were identical to Type I—including the extra density—the interface between the two Cs was a bit different than Type I filaments, rendering polymorphs at the ultrastructural level.
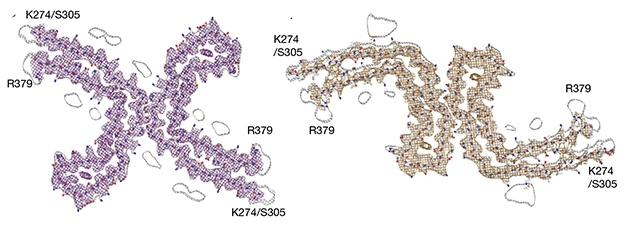
Ultrastructural Polymorphs. Type I (left) and Type II (right) filaments contain the same protofilaments, with different interfaces. [Courtesy of Falcon et al., 2019.]
What is this mysterious extra density? The scientists were unable to identify it, but they did narrow down the list. Because it is surrounded by hydrophobic side chains of tau, they concluded, it must be hydrophobic. It is not a protein, nor does it represent a post-translational modification of tau. In fact, though the density exists in one-to-one stoichiometry with tau, it shares no covalent bonds with tau. The researchers suggest that the molecule could form a rod-like structure that spans several tau protofilaments along their helical axis. Prime candidates include non-polar sterols and sterol derivatives, as well as fatty acids. Goedert speculated that the molecule could derive from axons damaged during head trauma, or even enter the brain from the blood when the blood brain barrier is compromised.
“Identification of the enigmatic molecules incorporated within CTE tau could yield clues to tau’s variable pathobiology, and possibly open new paths to diagnosis and treatment,” commented Lary Walker of Emory University in Atlanta. “Although the regional distribution of tauopathy in CTE can be explained, at least in part, by the physical effects of head injury on the brain, it is conceivable that the inclusion influences in some way the cellular and regional idiosyncrasies of CTE tauopathy.”
The researchers hypothesized that because the extra density is so abundant, and buried deep within the tau protofilament, it may incorporate into filaments as they form, or even help them form. However, Scheres noted that because the structure is an end product, it is impossible to tell if the extra density took advantage of CTE-tau’s already-open structure, or opened it up in the first place.
Khalid Iqbal of the New York State Institute for Basic Research in Developmental Disabilities favors the idea that CTE-tau’s distinctive structure derives from posttranslational modifications. He noted that several studies have demonstrated that changing tau’s phosphorylation or glycosylation patterns dramatically alters its structure and behavior in vivo.
Jucker wondered how these CTE filaments from end-stage disease compare to the filaments that form at the beginning, presumably when propagation happens. Scheres and Goedert said that the new structure could aid in the development of CTE-specific tau tracers, which would allow researchers to track the development of CTE-tau pathology as the disease progresses. They are currently working on resolving AD tau filaments coupled with tau tracers to understand how they bind.—Jessica Shugart
References
News Citations
Further Reading
Papers
- Fichou Y, Al-Hilaly YK, Devred F, Smet-Nocca C, Tsvetkov PO, Verelst J, Winderickx J, Geukens N, Vanmechelen E, Perrotin A, Serpell L, Hanseeuw BJ, Medina M, Buée L, Landrieu I. The elusive tau molecular structures: can we translate the recent breakthroughs into new targets for intervention?. Acta Neuropathol Commun. 2019 Mar 1;7(1):31. PubMed.
Primary Papers
- Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, Crowther RA, Newell KL, Ghetti B, Goedert M, Scheres SH. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019 Apr;568(7752):420-423. Epub 2019 Mar 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Emory University
This new report from the MRC Laboratory of Molecular Biology is yet another fascinating look at the molecular heterogeneity of polymeric tau. Tauopathy occurs in more than 20 different brain diseases (Spillantini et al., 2013), and thus may be the most common type of cerebral proteopathy. Even within the subset of tauopathies known as frontotemporal lobar degeneration–tau (FTLD-tau), the disease phenotype is quite variable (Dickson et al., 2011), but the source of variation, including selective regional and cellular vulnerability, remains uncertain.
Using cryo-electron microscopy (cryo-EM), Falcon and colleagues compared the molecular architecture of tau filaments from subjects who had died either with CTE or AD. This comparison is interesting because the tau that aggregates in the two disorders is similar, i.e., non-mutant tau consisting of all six isoforms of the protein. The researchers discovered that the molecular fold of tau in CTE differs from that in AD just enough to incorporate a mysterious molecular “rod” that runs within the long axis of the filament. This hydrophobic molecular inclusion could be a cofactor that influences the structure and possibly the pathobiology of aberrant tau; the authors speculate that it might consist of molecules such as non-polar sterols or fatty acids, but its true identity has yet to be revealed. In any case, it is apparent from this compelling cryo-EM analysis that polymeric tau in CTE differs structurally from that in AD.
One implication of the findings is the increasingly probable importance of biological cofactors in governing the structure and function of misfolded proteins in general. It has long been known that assemblies of recombinant or synthetic proteins often lack the seeding potency of the proteins that aggregate within the living organism (Jucker and Walker, 2018), although the in vivo seeding capacity (infectivity) of recombinant prion protein aggregated in vitro can be augmented by the inclusion of specific biomolecules during aggregation (Supattapone, 2014). As Falcon et al. note, identification of the enigmatic molecules incorporated within CTE tau could yield clues to tau's variable pathobiology, and possibly open new paths to diagnosis and treatment. Although the regional distribution of tauopathy in CTE can be explained, at least in part, by the physical effects of head injury on the brain, it is conceivable that the inclusion influences in some way the cellular and regional idiosyncrasies of CTE tauopathy.
Among the many questions to be addressed are the cause-and-effect relationship between the inclusion and the structure of tau molecules, the effects of the inclusion on the toxicity and seeding capacity of tau, and its impact, if any, on the pathobiology of oligomeric tau. Given the molecular similarity of tau in individuals with CTE and AD, it will be informative to determine why AD filaments lack the rod-like inclusions. And finally, one wonders whether such seemingly anomalous substances might occur in other proteinaceous fibrils.
References:
Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013 Jun;12(6):609-22. PubMed.
Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011 Nov;45(3):384-9. PubMed.
Jucker M, Walker LC. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat Neurosci. 2018 Oct;21(10):1341-1349. Epub 2018 Sep 26 PubMed.
Supattapone S. Synthesis of high titer infectious prions with cofactor molecules. J Biol Chem. 2014 Jul 18;289(29):19850-4. Epub 2014 May 23 PubMed.
New York Institute for Basic Research in Developmental Disabilities
This study clearly shows that CTE tau pathology is different from AD tau pathology and apparently each tauopathy has its own specific tau pathology signatures. I suspect that these different signatures of tau pathology in different tauopathies that show as different morphologies are very likely products of different posttranslational modifications. In 1996, we showed that de-glycosylation of AD neurofibrillary tangles (NFT)/paired-helical filaments converted them into straight filaments (Wang et al., 1996). Most recently, we showed that while AD phospho-tau injected in mouse hippocampus produces AD-like NFTs, AD p-tau partially dephosphorylated by PP2A produces argyrophilic grain-like tau pathology (Hu et al., 2016). Similarly, tau truncated at different sites and different combinations of 3R and 4R tau can produce tau pathology with different morphologies.
References:
Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med. 1996 Aug;2(8):871-5. PubMed.
Hu W, Zhang X, Tung YC, Xie S, Liu F, Iqbal K. Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers Dement. 2016 Oct;12(10):1066-1077. Epub 2016 Apr 28 PubMed.
Make a Comment
To make a comment you must login or register.