Tregs Suppress Astrogliosis and Improve Recovery After Stroke
Quick Links
In the weeks following a stroke, regulatory T cells stream into the brain. What exactly do they do there? Scientists led by Akihiko Yoshimura, Keio University School of Medicine, Tokyo, report in the January 2 Nature that these cells dampen astrogliosis that would otherwise damage neurons. The authors claim that these regulatory T cells (Tregs), and/or molecules they release, could point to new therapies for neuroinflammatory conditions, including Alzheimer’s and other neurodegenerative diseases.
- After stroke, infiltrating T cells drive down astrogliosis in the brain.
- T cells release amphiregulin, which calms astrocytes.
- Neural recovery improves.
“This is a very exciting paper and potentially important contribution to the literature,” said Stanley Appel, Houston Methodist Hospital. “We need effective therapies for the subacute phase of stroke. This is a promising mechanism to pursue.”
Previous studies have documented a rise in Tregs around the site of injury in mice two weeks after a stroke (Stubbe et al., 2013). To find out if these cells are beneficial, first author Minako Ito and colleagues induced ischemic stroke in eight- to 12-week-old mice and then tracked them for 60 days. As had been seen previously, a large number of Tregs began to appear in the brain 14 days after middle cerebral artery occlusion. Their number continued to rise, peaking around day 20 at about 2,000 cells per brain. When the scientists kept these Tregs out of the brain, either by preventing their migration or by killing them in mice engineered to express diphtheria toxin receptor in Tregs, the mice recovered only half as well as littermates with healthy Treg numbers.
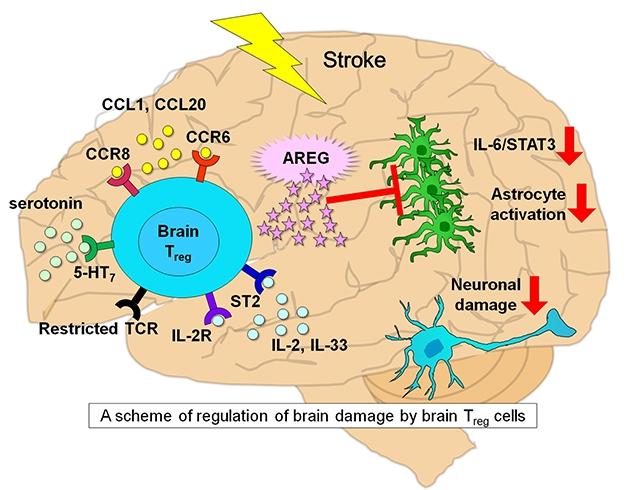
Tregs Jump into Action. Attracted by CCL1 and CCL20, Treg cells (turquoise) enter the brain after a stroke. They proliferate with the help of serotonin, IL-2R and ST2 receptor; produce the epidermal growth factor receptor ligand AREG; reduce astrogliosis; suppress pro-inflammatory cytokines IL6/STAT3; and limit neuronal damage. [Courtesy of Ito et al., 2019.]
When Tregs were depleted, astrocytes expressed more GFAP and neurotoxic genes, both characteristic of reactive astrocytes. On day 14, almost triple the number of neurons in motor cortices died compared with control mice that had normal Treg numbers. However, if the authors injected Treg cells into T-cell-deficient mice, then post-stroke astrogliosis returned to control levels and more neurons survived.
How do Tregs protect? According to transcriptional microarray analysis, the cells in the brain expressed genes typical for Tregs from other tissues, including the ST2 subunit of IL-33, PPARγ, and IL-10. However, they also expressed some genes typically found only in the nervous system, including the serotonin receptor type 7 (Htr7). Tregs taken from the stroke model mice proliferated in response to serotonin, implying a functional receptor. In vivo, both serotonin and selective serotonin reuptake inhibitors (SSRIs) increased Treg proliferation in the brain after stroke and sped up recovery.
It appears Tregs were recruited to the brain by chemokines CCL1 and CCL20, ligands for Treg receptors CCR8 and CCR6, respectively. Once in the brain, Tregs expressed amphiregulin, an epidermal growth factor receptor ligand. AREG calmed astrocytes even without Tregs, as an intraventricular AREG injection reduced astrogliosis and improved recovery. On the other hand, if mice lacked Tregs, their astrocytes expressed pro-inflammatory cytokines, such as IL-6 and STAT3.
Taken together, the results suggest that after stroke, Tregs are called to the brain, where they produce the AREG that controls astrogliosis. In mouse models of multiple sclerosis, Tregs performed similarly, suggesting that this mechanism could apply more broadly to neuroinflammatory diseases. In a separate recent study, restoring Tregs in the central nervous system slowed multiple sclerosis progression in mice (Nov 2018 news).
“We believe that our findings are important for improving neurological symptoms after stroke,” wrote Yoshimura to Alzforum. They hint that either enhancing the infiltration of Tregs into the brain or directly injecting them into the injured brain could improve outcomes, he added. In addition, he noted the data support findings from several other reports that SSRIs might be effective in stroke.
Michal Schwartz, Weizmann Institute of Science, Rehovot, Israel, believes the data have implications for a wide array of neurodegenerative diseases. She and others have reported that Tregs influence the rate of disease progression in amyotrophic lateral sclerosis, Alzheimer’s disease, and acute mental stress (Kunis et al., 2015; Baruch et al., 2015). “The results support the idea that we should harness the systemic immune system for brain repair,” said Schwartz.
Appel noted that not just astrocytes, but also microglia upped their expression of pro-inflammatory cytokines in the absence of Tregs. A similar analysis of microglia in these mouse brains might yield an additional response to AREG, he suggested.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Stubbe T, Ebner F, Richter D, Engel O, Randolf Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013 Jan;33(1):37-47. Epub 2012 Sep 12 PubMed.
- Kunis G, Baruch K, Miller O, Schwartz M. Immunization with a Myelin-Derived Antigen Activates the Brain's Choroid Plexus for Recruitment of Immunoregulatory Cells to the CNS and Attenuates Disease Progression in a Mouse Model of ALS. J Neurosci. 2015 Apr 22;35(16):6381-93. PubMed.
- Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat Commun. 2015 Aug 18;6:7967. PubMed.
Further Reading
Papers
- Liesz A, Kleinschnitz C. Regulatory T Cells in Post-stroke Immune Homeostasis. Transl Stroke Res. 2016 Aug;7(4):313-21. Epub 2016 Mar 31 PubMed.
- McCombe PA, Read SJ. Immune and inflammatory responses to stroke: good or bad?. Int J Stroke. 2008 Nov;3(4):254-65. PubMed.
Primary Papers
- Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019 Jan 2; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California
The authors propose an intriguing mechanism for T regulatory cell (Treg) involvement in tissue repair after brain injury. In this detailed report, Yoshimura and coworkers demonstrate Treg amplification dependent on interleukins-2 and -33, serotonin, and T cell receptor. Strikingly, they show that infiltration of Tregs into the ischemic brain is driven by the chemokines CCL1 and CCL20. In the absence of CNS Treg recruitment, astrogliosis and neurotoxicity become exacerbated in the middle cerebral artery occlusion stroke model. This report offers an insightful framework for how adaptive immunity in the form of Tregs referees immune function within the ischemic brain. Could this mechanism also operate in the context of AD? Quite possibly—investigating interaction(s) between Tregs, and perhaps other T cell subsets and amyloid-β/tau pathology, is clearly warranted.
Keio University School of Medicine
Thank you for your comments. We have already confimed that Tregs in the brains of AD model mice show brain Treg phenotypes. However, we are not sure whether brain Tregs in AD are good or bad for disease symptoms.
Make a Comment
To make a comment you must login or register.