Jumping Genes Rampant in Tau Flies
Quick Links
The human genome comes chock-full of transposable elements—ancient snippets of DNA that once hopped around the genome. Cells have evolved ways to silence these mobile motifs, but a new study suggests tau lets them loose, disrupting neuronal DNA and leading to a cell’s demise. Reporting July 23 in Nature Neuroscience, scientists led by Bess Frost, University of Texas Health San Antonio, find that human tau weakens epigenetic constraints and dampens the production of small RNAs that normally keep a lid on transposons. “Tau disrupts the two main cellular controls for silencing transposable elements in neurons,” said Frost. “We show that jumping transposable elements are deleterious to normal survival and causal for the disease process.” Tau may work the same way in people, Frost believes, as endogenous retroviruses similarly shake loose in tauopathy patients.
- Tau unfastens transposable elements (TE) from heterochromatin.
- Tau also depletes piRNAs that help degrade TE transcripts.
- Without either control, TE can jump and damage neurons.
“Until now, most of the evidence for the role of retrotransposons in neurodegenerative disease was in the context of ALS, multiple sclerosis, and macular degeneration,” said Joshua Dubnau, Stony Brook University School of Medicine, New York. “Now, this data suggests that loss of control of retrotransposons may be a hallmark of neurodegenerative disorders.” He cautioned that while activation of transposons may causally contribute to cellular toxicity and death, it could also be a consequence.
Transposable elements (TEs), also called transposons or “jumping genes,” are elements that can cut, copy, and paste themselves into different parts of the genome. Though TEs have persisted for millennia, most are transcriptionally silenced by constitutive heterochromatin. In other words, these genomic regions are so tightly wound on the chromosomes that they can’t jump. Any transcripts that do are caught by small RNAs called piwi-interacting RNAs. These bind and degrade TE transcripts before they can reintegrate in the genome.

The Model. Tau loosens heterochromatin and dampens piRNA production, leading to neuronal death. [Courtesy of Sun et al., Nature Neuroscience.]
Studies are finding that heterochromatin loosens with age, in cancer, and in TDP-43-mediated neurodegeneration, and this loosening accidentally releases the TEs (Li et al., 2013; Burns 2017; Li et al., 2012). Does tau free TEs, too? Frost’s group previously reported that tau relaxesd heterochromatin in flies, mice, and humans, and drove neuronal death in flies, but it was unclear if it also released transposable elements (Frost et al., 2014). Last month, another group of scientists led by Joshua Shulman, Baylor College of Medicine, Houston, reported that tau does release transposons, but these scientists didn’t know whether the transposons were reinserting back into the genome or driving neurodegeneration (Jun 2018 news).
First author Wenyan Sun and colleagues expressed, in Drosophila, either wild-type or mutated R406W human tau, which causes autosomal-dominant tauopathy. RNA sequencing showed that, compared with control flies, neurons in both transgenic flies started transcribing TEs by 10 days of age. Releasing more transposable elements caused neurons to reactivate their cell cycle, which had been demonstrated previously in tauopathy (Khurana et al., 2006).
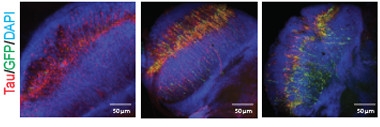
Active Transposons in Aging Neurons.
Fly retinal neurons (blue) that express human mutant tau (red) are normal on day one (left), but mobilize transposons (green) by day 10 (middle) that persist at day 40. [Courtesy of Sun et al., Nature Neuroscience.]
Could piRNAs quash the problem? In the transgenic flies, most of the piRNAs that were examined were expressed below control levels, as was piwi, their central regulator. Even in flies without human tau, reducing piwi activated the cell cycle and killed neurons, suggesting that having fewer piRNAs contributed to neurodegeneration. On the flip side, overexpressing piwi dampened transposable elements in the presence of tau, and fewer neurons died.
Did the unleashed transcripts reinsert into other spots? To find out, the authors used a gypsy-TRAP reporter, which makes neurons glow green when a transposable element lands in a particular chromosomal hot spot for reinsertions. Sure enough, more neurons glowed green in 10-day-old flies that expressed mutant human tau than in controls.
The authors were able to prevent these transposable elements from reintegrating and killing neurons by inhibiting reverse transcriptase with the drug 3TC. This enzyme makes a DNA copy from an RNA template to form a double-stranded helix that can reinsert into the genome. Also known as lamivudine, 3TC is FDA-approved for the treatment of HIV and hepatitis B. Feeding flies daily with 3TC-laced food normalized transposon expression and rescued neurons.
To see if tau might also free TEs in human neurons, the authors examined postmortem tissue from patients with AD and progressive supranuclear palsy. In both types of tissue, TEs, especially human endogenous retroviruses, were upregulated, suggesting that tau destabilizes the human genome as well, the authors wrote.
Together, the results suggest that tau, both wild-type and mutant, relaxes heterochromatin so that transposons are transcribed, and reduces the piRNAs that would normally keep them in check. Frost suspects this is a universal process, where tau causes heterochromatin to lose its hold across the genome rather than specifically affecting transposable elements. While this model only expresses tau in neurons, the effect likely hits all cells.
“I think this is going to be a fairly common finding in neurodegenerative diseases,” said Chris Link, University of Colorado Boulder. “The authors make a convincing case in their models that tau disrupts the heterochomatin.” He said he would need to see more data regarding tau’s effect on the levels of piwi protein. Transposable elements have been caught jumping in other diseases, but Frost hasn’t seen loosening of heterochromatin in other models. It could be that something else mobilizes transposons, he said.
“The underlying theme behind diseases of aging might be dysregulation of the transposable elements and the ability to keep DNA shut down,” said Avindra Nath, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland. “When that control is lost, there is activation of oncogenes, transposable elements, and retroviruses that wreak havoc.” The role of transposable elements has yet to be fully explored in neurodegenerative diseases, he said. “This draws attention to a part of the human genome that probably plays an important role in their pathophysiology.”
In future work, Frost wants to test whether endogenous retroviruses are reinserting into the human genome. If so, that would suggest that tau drives genome disruption and neurodegeneration in people. In that case, a drug like 3TC could theoretically work in AD. Certain antiretrovirals are currently in clinical trials for amyotrophic lateral sclerosis, as TDP-43 also reportedly frees human endogenous retroviruses (Oct 2015 news).
Genome disruption and cell cycle activation may not be the only mechanisms of cell death. Transposable elements are by nature repetitive and so can form double-stranded RNA (dsRNA), the authors noted. The presence of dsRNA sets off alarm bells in the innate immune system, which could contribute to neuroinflammation, they wrote.
Link noted that relaxed heterochromatin likely releases other repetitive elements in the genome, which might contribute to an inflammatory response. A recent paper spotted more transcripts for viral response genes in AD than control brain (Jun 2018 news). DsRNA is a bridge between these two papers, Link said, as it crops up with both endogenous and infectious viruses.—Gwyneth Dickey Zakaib
References
News Citations
- Tau Aggregates Awaken Genetic Relics in the Brain
- Do Sleeper Viruses Awaken in ALS?
- Aberrant Networks in Alzheimer’s Tied to Herpes Viruses
Paper Citations
- Li W, Prazak L, Chatterjee N, Grüninger S, Krug L, Theodorou D, Dubnau J. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci. 2013 May;16(5):529-31. Epub 2013 Apr 7 PubMed.
- Burns KH. Transposable elements in cancer. Nat Rev Cancer. 2017 Jul;17(7):415-424. Epub 2017 Jun 9 PubMed.
- Li W, Jin Y, Prazak L, Hammell M, Dubnau J. Transposable elements in TDP-43-mediated neurodegenerative disorders. PLoS One. 2012;7(9):e44099. Epub 2012 Sep 5 PubMed.
- Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014 Mar;17(3):357-66. Epub 2014 Jan 26 PubMed.
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006 Feb 7;16(3):230-41. PubMed.
Further Reading
Primary Papers
- Sun W, Samimi H, Gamez M, Zare H, Frost B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci. 2018 Aug;21(8):1038-1048. Epub 2018 Jul 23 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Baylor College of Medicine / Texas Children's Hospital
In this independent and insightful study combining investigations in fruit flies and human postmortem tissue, Dr. Frost’s team reports results that agree with, and significantly add to, our recent findings that tau pathology can modulate the activity of transposable elements in Alzheimer’s disease.
This work addresses several questions that remained unresolved by our work. First, they used an available endogenous reporter to demonstrate additional evidence supporting new transposon insertions activated by tau. Second, Frost and colleagues enhance our understanding of relevant mechanisms, showing that besides chromatin relaxation, tau induced a reduction in piwi-interacting RNAs. Further, genetic manipulations that interfere with piRNAs enhance both TE expression and tau-induced neurotoxicity. Lastly, other interventions, including dietary restriction and pharmacologic treatment with the reverse transcriptase inhibitor 3TC, suppressed both TE activity and tau-induced neuronal death.
While this work is highly suggestive, in my view, a key remaining question is whether TE activity truly directly mediates tau-induced neuronal injury or alternatively might yet represent a non-causal biomarker. This is a very challenging question to answer because most experimental manipulations evaluated, e.g. piRNA modulation, chromatin modulation, dietary restriction, and reverse transcriptase inhibitors, have widespread cellular and organismal impact beyond that of TE suppression.
Regardless, I commend Dr. Frost and colleagues on this comprehensive study. It will surely inspire additional investigations of this important topic.
University of Delhi South Campus
We have previously reported heterochromatin decondensation and global transcriptional upregulation, affecting both protein coding and non-oding genes, in Drosophila tauopathy models. Moreover, we believe that tau-induced heterochromatin decondensation is a leading factor that seeds neurotoxicity and disease pathogenesis.
Sun et al. here report an increase in transcripts of the retrovirus group of transposable elements and transposable element dysregulation due to heterochromatin decondensation in tau expressing Drosophila brain cells. Based on the above and other experimental evidence, the authors propose that reverse transcriptase inhibitors could be used as a potential therapeutic strategy for the treatment of neurodegenerative tauopathies, including Alzheimer’s.
Though transposon dysregulation has been noted earlier in some neurodegenerative disorders, this is an interesting finding and may have some future application potential. However, tau-induced chromatin remodeling and global transcriptional upregulation is likely to (in fact it does) dysregulate a large number of genes, especially genes located near heterochromatic regions. Hence, an increase in transcripts of the retrovirus group of transposable elements could be one or a few of them.
Therefore, dysregulation of the retrotransposon genes and aberrant hopping of transposable elements in the genome of tau-expressing neuronal cells could be a fraction of more global happenings. Also, it is not clear if transposon dysregulation and heterochromatin decondensation form a vicious loop and regulate each other, or which appears first.
Taken together, this is an interesting finding that shows a consequence of the global transcriptional upsurge. To me, it is the tip of the iceberg.
Make a Comment
To make a comment you must login or register.