Aberrant Networks in Alzheimer’s Tied to Herpes Viruses
Quick Links
An extensive, multi-cohort, multi-network analysis of what goes wrong in the Alzheimer’s brain and why, has turned up none other than human herpes and other viruses. The big data analyses, a four-year project, is detailed in the June 21 Neuron. Led by Joel Dudley at the Icahn School of Medicine at Mount Sinai, New York, the authors can’t be sure if the microbes are causing molecular and genetic networks to run amok or are merely taking advantage of the disruption to flex their minuscule genomes. Even so, other scientists say the findings are cause for a rethink.
- Viral load linked to activation of gene networks in preclinical AD.
- Viruses may be driving production of Aβ.
- Is viral reactivation a cause or consequence of AD pathology?
“This is in line with the now-rapidly increasing evidence of viral infections as key drivers in the development of Alzheimer’s disease pathology,” wrote Hugo Lövheim, Umeå University, Sweden. The new findings tie viral load to increased expression of components of the amyloid pathway in people with preclinical AD. This fits with the related hypothesis that Aβ normally functions as an endogenous antimicrobial defense, Lövheim notes (full comment below). On that note, an upcoming paper in the same journal reports that Aβ fibrils neutralize herpesviruses in cells and animals. Rudy Tanzi and Robert Moir at Massachusetts General Hospital, Charlestown, co-led this second study, currently available as a “sneak peek” on Cell Press. “The combined story might be the beginning of a paradigm shift, in which low-grade, subclinical infection, e.g., by herpes-type viruses and other microbes, could seed plaques,” wrote Tanzi.
Others were more cautious. Charlotte Warren-Gash, from the London School of Hygiene and Tropical Medicine, noted that robust longitudinal population data sets are needed to assess the clinical relevance and generalizability of such findings, and how they relate to the trajectory of Alzheimer’s disease. John Hardy of University College London noted that the work is difficult to square with the occurrence of Alzheimer’s disease in Down’s syndrome and in all carriers of some APP and PSEN mutations (full comment below).

Viruses and AD. Multi-omic evidence links viral load to the molecular, pathological, and clinical manifestations of dementia. [Courtesy of Ben Readhead.]
Alzheimer’s has long been linked to viruses. In 1997, Ruth Itzhaki and colleagues at the University of Manchester, U.K., reported that, when present in the brain, the cold sore virus herpes simplex 1 (HSV-1) was a strong risk factor for the disease, and later the scientists located HSV-1 in amyloid plaques (Itzhaki et al., 1997; Wozniak et al., 2009). They also found that AD brains had much more human herpesvirus 6 (HHV-6) than did controls (Lin et al., 2002).
In longitudinal studies, Lövheim and colleagues reported that people with anti-HSV-1 antibodies in their blood were at higher risk for incident AD (Lövheim et al., 2014; Lövheim et al. 2014). Others have linked Epstein-Barr, cytomegalovirus, and herpes viruses to AD, as well (e.g., Carbone et al., 2014).
Recently, nationwide population-based studies in Taiwan reported that varicella zoster virus and HSVs are risk factors for dementia, and that antivirals can reduce that risk—in the case of HSVs by 80 percent (Chen et al., 2018; Tzeng et al., 2018; for commentary, see Itzhaki and Lathe, 2018). These population studies are important, said Itzhaki. “Proof of a microbe being a cause of a noninfectious disease can be shown by either treating with an agent that specifically targets the microbe, or by preventing it with a specific vaccine. Despite shortcomings, the Taiwan studies are essential first steps,” Itzhaki wrote to Alzforum.
That these viruses are common makes potential connections to dementia troubling. By some estimates, 90 percent of the population carries HSV-1 by age 70, and almost everyone is infected with HHV-6 in the first three years of life. These viruses can lie dormant for decades.
Despite the apparently ubiquitous threat, the Alzheimer’s field thus far has largely dismissed links to viruses (Feb 2011 webinar). Lack of mechanistic explanations have seeded doubt. “Previous studies of viruses and Alzheimer’s have been very indirect and correlative,” noted Dudley, who splits his time between Mount Sinai and the ASU-Banner Neurodegenerative Disease Research Center at Arizona State University in Tempe. The new work attempts to change that. “We performed a sophisticated computational analysis using multiple levels of genomic information measured directly from affected brain tissue, which allowed us to identify how the viruses directly interact with, or co-regulate, known Alzheimer’s genes.”
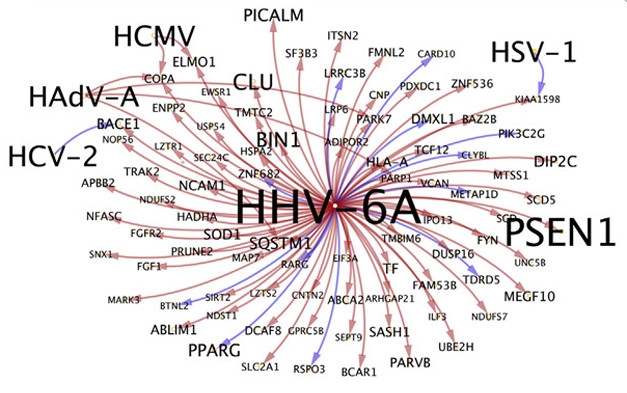
Upregulated Host Genes. Host genes upregulated by the HHV-6A virus include ones linked to AD, including PSEN1, CLU, BIN1, and PICALM. [Courtesy of Readhead et al., Neuron 2018.]
Joint first authors Ben Readhead, Jean-Vianney Haure-Mirande, and colleagues built on molecular network analysis pioneered by Bin Zhang and co-author Eric Schadt, both also from Mount Sinai (May 2013 webinar on Zhang et al., 2013). Readhead is now at ASU-Banner Neurodegenerative Disease Research Center at Arizona State University, Tempe.
The scientists started out by looking for differences among gene-regulatory networks in both cognitively and pathologically healthy controls and people with preclinical AD. For that they used a data set created by researchers at the Translational Genomics Institute, Phoenix. Freely shared raw data was the foundation for a lot of the work in this Neuron paper. “It’s a success story for the idea of open data access and sharing data in its most raw form,” Readhead emphasized.
The preclinical samples were from people who had moderate neurofibrillary tangle pathology—Braak Stage II to IV—and moderate plaques by CERAD score, but no evidence of cognitive impairment (Liang et al., 2010). Readhead and colleagues focused on gene expression in areas known to be subject to profound neuronal loss in AD, namely the entorhinal cortex and the hippocampus.
The researchers first built probabilistic causal networks. In other words, by comparing control and preclinical disease samples, they identified groups of related genes likely to be involved in the disease process at this stage. To get a handle on what regulates those groups, they identified key drivers, i.e. genes that likely control the activity of large subnetworks within a given network.
Perhaps unsurprisingly, genes previously linked to AD popped up among the drivers, including APP, PICALM, and ABCA1. But here, also, is where the first hints of viral involvement emerged. When the scientists looked at the sorts of biological mechanisms that could explain the differences between the key drivers in each network, Readhead and Haure-Mirande realized that they shared a set of DNA motifs, i.e., sequences that bind a specific set of transcription factors known as C2H2 zinc fingers. C2H2-TFs have been implicated in viral biology. For example, the C2H2-TF SP1 binds Epstein-Barr virus proteins, regulates transcription of HIV genes, and promotes replication of human cytomegalovirus. This pointer to viral involvement emerged from an unbiased analysis.
Could viruses be driving causal networks in AD? There were other hints. “We had a laundry list of reasons why we thought this might be the case and got to the point where we thought we should really look into this carefully,” Readhead told Alzforum. “Once we realized we had a signal, the essential goal became to look across as many perspectives as possible for how viruses interact with AD biology. For example, is it a specific virus, group of viruses, or pan virus effect, i.e., just the fact of having a viral infection? The paper builds up these perspectives.”

Viruses and Plaques.
In the absence of miR-155 (left), which is suppressed by HHV-6A, amyloid plaques (red) grow larger than normal (right). Microglia are green. [Courtesy of Readhead et al., Neuron, 2018.]
For that, the researchers capitalized on the Accelerated Medicines Partnership-AD project. Coordinated by the NIH, this public-private partnership funds multi-omic profiling of large data sets. Readhead and colleagues started with samples from the Mount Sinai Brain Bank, looking for correlations between AD and viral RNA and DNA. HHV-6A and HHV-7 tended to be more abundant in the anterior prefrontal cortices (213 total samples) and superior temporal gyri (96 samples) of people with AD, likely AD, and possible AD than in controls. In some cases and brain regions, other viruses negatively correlated. For example, human adenovirus B1 and HHV-6B were more abundant in the anterior prefrontal cortices of controls than of cases. Readhead thinks expression of some viruses might compete with expression of others.
The correlation extended to individual viral elements, such as repeats that flank viral genes, and to other cohorts. In 151 temporal cortex samples from the Mayo Clinic and 247 dorsolateral prefrontal cortex samples from the Religious Orders Study at Rush University, Chicago, more HHV-6A and HHV-7 were found in people with probable AD than controls.
The correlation didn’t always hold. No difference in viral load emerged between AD and control samples from the Memory and Aging Project at Rush. Nonetheless, Chris Gaiteri at Rush found the cohort replication compelling. “Keep in mind that these cohorts are selected in different ways and from different parts of the country. If you don’t see replication it does not necessarily mean that your original observation is wrong, but to see replication in three different cohorts is pretty impressive,” Gaiteri said. Furthermore, viral load correlated with neuropathological and clinical facets of disease. Expression of certain HHV-6A genes in the prefrontal cortex correlated with clinical dementia rating, amyloid plaque density, and Braak score.
What could these viruses be doing in the AD brain? To address this, the authors turned to viral quantitative trait loci. These are host genetic variants that associate with viral abundance. They found 1,672 vQTLs across four brain regions that associated with 16 viruses. Once again, HHV-6A stood out, having 103 vQTL markers. These included genes involved in mucosal immunity, innate immunity, and viral sensing. For each virus, the authors found multiple vQTLs that associated with AD genetic risk variants, CDR score, or AD neuropathology. “These results indicate a significant overlap between the genetic basis for AD traits and the abundance of specific viral species and viral genomic features,” write the authors.
The researchers took it a step further. By setting vQTLs as “causal anchors,” they constructed directional relationships between viral species and host genes. In this way they built a new set of networks. These now are networks of host genes that regulate, or are regulated by, each virus in any of four different brain regions: the anterior prefrontal cortex, superior temporal gyrus, parahippocampal gyrus, and inferior frontal gyrus. By this analysis, three viruses—HSV-1, HSV-2, and HHV-6A—controlled host genes in all four regions.
Genes most commonly regulated by the viruses included BACE1, Fyn kinase, and PPAR-γ, all of which have been implicated in AD. For HHV-6A specifically, the host genes purportedly regulated by the virus had considerable overlap with AD and included genes involved in APP processing and Aβ metabolism, such as presenilin 1, clusterin, Bin1, and PICALM (see image above). Others are known to mediate immune responses to viruses. Fyn promotes interferon-γ1 production during viral infection, while PPAR-γ is known to suppress viral replication. Links between other viruses and other AD genes also emerged. The authors found that HAdV-C-induced expression of complement receptor 1, and that HSV-2 inhibited TOMM40.
All told, the findings hint that viruses that have lain dormant in the brain for decades may be active in AD, or that there might be some form of chronic low-grade infection that goes undetected.
Is this a cause or a consequence of the disease? “That’s the most important question,” said Readhead. “Are these viruses opportunistic bystanders in a compromised host, or do they accelerate the disease once the brain becomes dysfunctional?” Readhead suspects the latter. “I think it’s plausible that they impact how quickly disease progresses once established, though we don’t know for certain,” he said. “The striking overlaps between the sets of genes that viruses perturb and AD risk genes and APP processing genes are too compelling to dismiss.”
There is no love lost between neurons and viruses. Even outside of age-related neurodegeneration, the tiny parasites cause nasty pathology, from shingles to temporary paralysis and even fatal encephalitis. Among the different cell types in the brain, HHV-6A might affect neurons directly. When Readhead and colleagues used quantitative RNA-Seq analysis to tease out relationships between viral load, AD trait, and the abundance of specific cell types, they found that viruses correlated with loss of neurons, particularly in the superior temporal gyrus. They traced this to specific networks of genes, including suppression of the gene encoding miR-155, a micro RNA known for its neuroprotective effects. When they crossed miR-155 knockouts with APP/PS1 mice and tested four-month-old offspring, they saw that plaques were larger and more numerous than in APP/PS1 controls. The work suggests that viruses could directly affect plaque growth.
That fits with Tanzi and Moir’s idea of Aβ being antimicrobial. Previously, they reported that the peptide erects a physical barrier against invading bacteria and fungi, cocooning them in amyloid fibrils (May 2016 news). Others have reported that Aβ40/42 can prevent HSV-1 entry into cells (Bourgade et al., 2015). Now, the MGH researchers report in their upcoming paper that Aβ can bind an agglutinate HSV-1 and HHV-6 in cells and in mice, preventing acute encephalitis. Whether host networks have evolved to mobilize in response to viral threats by upping production of Aβ remains unknown.
How about therapeutic implications? Don’t reach for the Valtrex yet. It remains to be seen how viral load correlates with disease progression. “As Readhead et al. note, we cannot know the cognitive health trajectories of the ‘preclinical AD’ patients, and disentangling molecules involved in disease progression from those responsible for maintenance of brain function is complex,” noted Warren-Gash. “Linking electronic health records and –omics data may provide insights into potentially tractable mechanisms of AD pathogenesis, but many questions remain about when, how, and in whom interventions would be indicated,” she added (full comment below). In years past, Itzhaki has applied for funding for anti-viral AD clinical trials but was turned down.—Tom Fagan
References
Webinar Citations
- Herpes Simplex and Alzheimer’s—Time to Think Again?
- Can Network Analysis Identify Pathological Pathways in Alzheimer’s
News Citations
Paper Citations
- Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997 Jan 25;349(9047):241-4. PubMed.
- Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009 Jan;217(1):131-8. PubMed.
- Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer's disease. J Pathol. 2002 Jul;197(3):395-402. PubMed.
- Lövheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimers Dement. 2015 Jun;11(6):593-9. Epub 2014 Jul 17 PubMed.
- Lövheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F. Herpes simplex infection and the risk of Alzheimer's disease-A nested case-control study. Alzheimers Dement. 2014 Oct 7; PubMed.
- Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, Licastro F. Herpes virus in Alzheimer's disease: relation to progression of the disease. Neurobiol Aging. 2014 Jan;35(1):122-9. PubMed.
- Chen VC, Wu SI, Huang KY, Yang YH, Kuo TY, Liang HY, Huang KL, Gossop M. Herpes Zoster and Dementia: A Nationwide Population-Based Cohort Study. J Clin Psychiatry. 2018 Jan/Feb;79(1) PubMed.
- Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, Lu RB, Chang HA, Kao YC, Yeh HW, Chiang WS, Chou YC, Tsao CH, Wu YF, Chien WC. Anti-herpetic Medications and Reduced Risk of Dementia in Patients with Herpes Simplex Virus Infections-a Nationwide, Population-Based Cohort Study in Taiwan. Neurotherapeutics. 2018 Apr;15(2):417-429. PubMed.
- Itzhaki RF, Lathe R. Herpes Viruses and Senile Dementia: First Population Evidence for a Causal Link. J Alzheimers Dis. 2018;64(2):363-366. PubMed.
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013 Apr 25;153(3):707-20. PubMed.
- Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA. Neuronal gene expression in non-demented individuals with intermediate Alzheimer's Disease neuropathology. Neurobiol Aging. 2010 Apr;31(4):549-66. PubMed.
- Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fülöp T Jr. β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology. 2015 Feb;16(1):85-98. Epub 2014 Nov 7 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, Reiman EM, Schadt EE, Ehrlich ME, Gandy S, Dudley JT. Multiscale Analysis of Independent Alzheimer's Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron. 2018 Jul 11;99(1):64-82.e7. Epub 2018 Jun 21 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Edinburgh
University of Edinburgh
Microbes and Alzheimer's Disease: Paradigm Change
In this article Readhead et al. provide fresh impetus to the link between infection and Alzheimer’s disease (AD). A causal link has long been suspected (Itzhaki et al., 2016), underlined by the finding that the signature of AD, Aβ, is now established as an antimicrobial defense peptide (Soscia et al., 2010; Kumar et al., 2016).
Because the majority of the adult population already harbors subclinical infections with diverse viruses, notably including herpes simplex viruses types 1 and 2 (HSV-1/2), as well as human herpes viruses 6A, 6B, and 7 (HHV-6A/6B/7), and others such as Epstein–Barr virus (EBV), a declining immune system with age might permit reactivation of erstwhile silent viruses, exacerbating pathology from another cause, but playing no causal role. In the alternative, such viruses might be essential triggers.…More
Herpesviruses: Evidence for Causation
To place this in context, evidence has recently emerged directly and causally linking infection with HSV-1, and possibly by HSV-2, to AD development. Briefly, population data from Taiwan argue that 10-year AD development could be prevented, in 90 percent of treated patients, with aggressive antiviral medication at the time of acute herpes infection (Tzeng et al., 2018; Itzhaki and Lathe, 2018). This work establishes beyond reasonable doubt that herpesviruses can cause AD, as pioneered by Itzhaki (Itzhaki et al., 1997), but raises two key questions: (i) which herpesvirus? and (ii) how many cases? The new paper by Readhead et al. tackles both questions head-on.
The New Results: Overabundance of HHV in AD
To date most studies have focused on HSV-1. HSV-1 DNA is widely found in AD brain, as well as in control tissue, and association with Aβ plaques in AD brain has been demonstrated (Wozniak et al., 2009). By contrast, other herpesviruses such as HHV-6 have been predominantly implicated in multiple sclerosis (Leibovitch and Jacobson, 2014), and not so far in AD. The striking new results from Readhead et al. cut across this compartmentalization. In a long and often complex paper, they first report that transcripts of multiple herpesviruses are increased in AD brain—predominantly HHV-6A, HHV-6B, and HHV-7, although there was also evidence of over-representation of HSV-1 and HSV-2 transcripts. Notably, the overabundance was not restricted to a few odd cases, but from the data at hand appears to be a more general feature of AD. Unfortunately, other potential AD-linked pathogens including spirochetes (Miklossy, 2015) do not appear to have been systematically surveyed. Second, DNA variants involved in innate immunity and antiviral responses (that have previously been highlighted as risk factors for AD) were found to correlate with viral abundance and degree of AD pathology. Third, they show that there was also increased abundance of HHV-6A DNA, pointing to active viral replication in AD brain. Further work in this exciting compendium of results, including network and multivariate analysis, highlighted HHV-6A and HHV-7 as central associates of AD development. The authors rightly conclude that their data support an important role of these viruses, particularly HHV-6A and HHV-7, in AD development.
Like HSV-1/2, both virus types have a very high prevalence of more than 80–90 percent in adults worldwide (Tanaka-Taya et al., 1996). Moreover, similarly to HSV-1/2, HHV-6 and -7 are well-known causes of viral encephalitis, in particular in immunocompromised individuals, and have also been shown to be associated with demyelinating brain diseases (Pietiläinen-Nicklén et al., 2014). In contrast to the eight other known human herpesviruses, HHV-6A and 6B integrate into the host genome with a very high frequency in both somatic and germline cells and can thus be inherited. The detection of HHV-6A/B or HHV-7 DNA is therefore not necessarily a sign of active infection, only the expression of lytic HHV-6A/B or HHV-7 transcripts is indicative of this, as in the study by Readhead and colleagues.
In a very recent addition to the debate, Moir and colleagues (Eimer et al., 2018), upcoming also in Neuron, confirm that the AD signature protein, Aβ, binds to HSV-1 and HHV-6 surface glycoproteins, and mediates agglutination and protection against virus challenge, further reinforcing the link between herpesviruses, Aβ, and AD.
Drivers or Passengers? Differential Tropism Could Point the Way Forward
Readhead et al. highlight the challenge ahead: “Distinguishing the earliest drivers of disease from the ‘opportunistic passengers’ of a multi-decade neurodegenerative process is especially formidable ...” In other words, herpesvirus infection of affected brain areas might either be a causal component/cofactor for AD or a consequence of the tissue alterations in the affected AD areas. An important component that has to be considered is the frequently observed invasion of inflammatory immune cells in these areas. We suggest that the differential tropism of the viruses might offer an insight.
Whereas HSV-1 and HSV-2 are considered to be “neurotropic,” in that they have a predilection to infect and replicate in neurons, the HHV-6A/B and HHV-7 as a group have generally been dubbed “lymphotropic” in that they principally target immune cells, including T cells and macrophages, and also are reported to infect glial cells e.g., oligodendrocytes. Work has been done on identifying receptors for HSV-1 and mapping them in human brain (e.g., Lathe and Haas, 2017), but much less is known about receptors and co-receptors for HHVs. None of the candidates identified so far—CD4, CD43, and CD134/TNFRSF4—alone permits infection by HHVs, noting that herpesviruses are unusual in that they require a cluster of host receptors for effective infection; a single receptor is insufficient. Therefore our conclusions are tentative. Indeed, the terms neuro- and lymphotropic are inaccurate. However, they serve to illustrate the potential for reciprocal interactions among HHV-6A/B, HHV-7, and HSV-1/2, in vivo, via several different pathways.
AD is accompanied by a major CNS influx of proinflammatory immune cells, including macrophages (e.g., Fiala et al., 2002), to boost pathogen elimination. Invading cells harboring episomal or integrated HHV genomes might have skewed the AD versus non-AD ratio of viral transcripts detected by Readhead et al. It would thus be very interesting to study the distribution of HHV genomes in neuronal versus non-neuronal cells.
In addition, active herpesvirus infections can foster reactivation of other latent herpesviruses: for example, human cytomegalovirus infection is accompanied by reactivation of latent HSV-1 (Stowe et al., 2012). In this regard a 2016 paper by Chapenko et al. (Chapenko et al., 2016) is notable. Briefly, in this paper some 30–80 percent of all human brain samples studied (controls and “unspecified encephalopathy,” UCP, no precise diagnosis provided; mean age in both cases 58–59 years) were positive, as expected, for both HHV-6 and HHV-7 (HHV-6A and HHV-6B were not separately subtyped), and there was no significant difference in positivity between control and UCP. By contrast, in UCP frontal and temporal lobe there was a 100-fold, and highly significant, increase in HHV-6 genome content (Chapenko et al., 2016). Despite diagnostic caveats, it is clear that some event or events in these individuals switched on HHV replication—perhaps by genome reactivation induced by another triggering factor/agent, or by an influx of susceptible cells, or both.
Conclusions: Rethinking Alzheimer’s Disease from Scratch
Immense effort has been expended on targeting Aβ, without any success, and it now turns out that it is a defense molecule. The Readhead paper, combined with Tzeng et al. (Tzeng et al., 2018) and Eimer et al. (Eimer et al., 2018), is driving a paradigm change. Viruses are steadily moving to the fore as vital contributors to AD development, but which virus? Or all? Or are they all opportunist infections of a degenerating brain?
Given that there is causal evidence for an involvement of HSV-1 in AD (Tzeng et al., 2018; Itzhaki and Lathe, 2018), and a recent paper from the team at Genentech reveals that a receptor gene for HSV-1 (PILRA, encoding a receptor that is probably not targeted by HHVs) is a significant risk factor for AD (Rathore et al., 2018), it seems that HSV-1 is likely to play a direct role. However, it could well be that HSV-1 brings in HHV by immune cell recruitment and/or reactivation, leading to “double pathology.” The Readhead paper is a convincing demonstration that HHVs are also likely to play a role.
But what is it that first triggers AD? It does not seem to be infection per se, because these viruses are everywhere. Key “AD genes” encoding immune system modulators such as APOE are clearly important (and APOE alleles modulate susceptibility to diverse pathogens including herpes viruses, HIV, Chlamydia, and malaria), and lifestyle factors such as stress may play a role. Squinting ahead, it could be that a combination of infection, genes, age, and environment might explain a majority of AD cases.
However, lest we spend too much time peering into the mist, we should focus on what we know. As it stands, the field has established that viruses are somewhere central in the causal chain. The intellectual property of many antivirals, such as aciclovir as the first-line drug against HSV-1/2 and another herpes virus, varicella-zoster virus (VZV), is no longer protected, and interest from pharmaceutical companies will probably be limited. But is that a good reason not to follow-up? To date, hundreds of drugs against AD have failed in clinical Phase 1-3 studies. Although these studies cost billions of dollars, not a single drug has been successful. We believe that the increasing evidence over the past few years—including the paper by Readhead and colleagues—that chronic infections and inflammatory processes are central to AD, clearly warrants revisiting aciclovir and other antiviral drugs (as well as vaccination) as potential routes to combating AD.
Moreover, we should not limit ourselves to herpesviruses (both spirochetes and fungi have been associated with AD, indeed Readhead et al. detected traces of diverse pathogens such as human adenovirus, Ippy arenavirus, Torque teno virus, and Kaposi sarcoma-associated herpesvirus in AD brain). Nor should we overlook other disorders, Parkinson’s disease for example, where an infectious etiology has long been speculated.
References:
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WS, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA. Microbes and Alzheimer's Disease. J Alzheimers Dis. 2016;51(4):979-84. PubMed.
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010 Mar 3;5(3):e9505. PubMed.
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016 May 25;8(340):340ra72. PubMed.
Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, Lu RB, Chang HA, Kao YC, Yeh HW, Chiang WS, Chou YC, Tsao CH, Wu YF, Chien WC. Anti-herpetic Medications and Reduced Risk of Dementia in Patients with Herpes Simplex Virus Infections-a Nationwide, Population-Based Cohort Study in Taiwan. Neurotherapeutics. 2018 Apr;15(2):417-429. PubMed.
Itzhaki RF, Lathe R. Herpes Viruses and Senile Dementia: First Population Evidence for a Causal Link. J Alzheimers Dis. 2018;64(2):363-366. PubMed.
Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997 Jan 25;349(9047):241-4. PubMed.
Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009 Jan;217(1):131-8. PubMed.
Leibovitch EC, Jacobson S. Evidence linking HHV-6 with multiple sclerosis: an update. Curr Opin Virol. 2014 Dec;9:127-33. Epub 2014 Oct 17 PubMed.
Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer's disease. Front Aging Neurosci. 2015;7:46. Epub 2015 Apr 16 PubMed.
Tanaka-Taya K, Kondo T, Mukai T, Miyoshi H, Yamamoto Y, Okada S, Yamanishi K. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J Med Virol. 1996 Jan;48(1):88-94. PubMed.
Pietiläinen-Nicklén J, Virtanen JO, Uotila L, Salonen O, Färkkilä M, Koskiniemi M. HHV-6-positivity in diseases with demyelination. J Clin Virol. 2014 Oct;61(2):216-9. Epub 2014 Jul 21 PubMed.
Lathe R, Haas JG. Distribution of cellular HSV-1 receptor expression in human brain. J Neurovirol. 2017 Jun;23(3):376-384. Epub 2016 Dec 15 PubMed.
Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002 May;32(5):360-71. PubMed.
Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Reactivation of herpes simplex virus type 1 is associated with cytomegalovirus and age. J Med Virol. 2012 Nov;84(11):1797-802. PubMed.
Chapenko S, Roga S, Skuja S, Rasa S, Cistjakovs M, Svirskis S, Zaserska Z, Groma V, Murovska M. Detection frequency of human herpesviruses-6A, -6B, and -7 genomic sequences in central nervous system DNA samples from post-mortem individuals with unspecified encephalopathy. J Neurovirol. 2016 Aug;22(4):488-97. Epub 2016 Jan 4 PubMed.
Rathore N, Ramani SR, Pantua H, Payandeh J, Bhangale T, Wuster A, Kapoor M, Sun Y, Kapadia SB, Gonzalez L, Zarrin AA, Goate A, Hansen DV, Behrens TW, Graham RR. Paired Immunoglobulin-like Type 2 Receptor Alpha G78R variant alters ligand binding and confers protection to Alzheimer's disease. PLoS Genet. 2018 Nov;14(11):e1007427. Epub 2018 Nov 2 PubMed.
Umeå University
This work by Readhead and colleagues is an important contribution to the field. The authors have performed a really impressive, in-depth analysis of postmortem Alzheimer’s disease brain tissue samples using modern bioinformatics techniques, and found multiple lines of evidence linking viral infections, in particular human herpes virus 6A and 7, to Alzheimer’s disease.
This is in line with the now-rapidly increasing evidence of viral infections as key drivers in the development of Alzheimer’s disease pathology. In recent years, it has been established that the Aβ peptide accumulating in Alzheimer’s disease amyloid plaques is a potent antimicrobial peptide and a key constituent of the brain’s innate immune defense against multiple pathogens. An emerging understanding of these findings could be that persistent viral infections within the brain could trigger this immune response over prolonged periods of time, resulting in pathological Aβ accumulation and eventually progressive cell death. The linking of known risk genes of Alzheimer’s disease to the immune defense and viral infections, as in the current work, provides further substance to this understanding of the drivers of the disease process.…More
HHV6A is a ubiquitous pathogen, with nearly 100 percent of the population being infected; however, this primarily T-cell-associated virus seldom causes symptoms in immunocompetent adults. A limitation of studies of postmortem brain material is that the finding of pathogen associations might represent late superinfection of the diseased brain rather than true early inducers of the pathological processes.
Until today, only herpes simplex has been confirmed to increase the risk of later Alzheimer’s disease development in large prospective population-based materials. With that said, I think the current and previous results indicate a role of HHV6A, at least in late Alzheimer’s disease, and I think future clinical antiviral drug trials to target Alzheimer’s disease development should seek to cover both HSV1 and HHV6A/HHV7.
London School of Hygiene and Tropical Medicine
This is an interesting paper comparing postmortem brains of individuals with either AD neuropathology but no cognitive change (“preclinical AD”) or clinical AD, to brains of normal individuals. Subjects with clinical AD had higher levels of human herpesvirus-6A and -7. Viral abundance was associated with various modulators of amyloid precursor protein metabolism.
However, no epidemiological data was presented on individuals who took part in these postmortem brain studies. As Readhead et al. note, we cannot know what the cognitive health trajectories of the “preclinical AD” patients would have been, and therefore disentangling molecules involved in disease progression from those responsible for maintenance of brain function is complex. Robust longitudinal population data sets are needed to assess the clinical relevance and generalizability of such findings, and how they relate to disease trajectory. In future, linking electronic health records and –omics data may provide insights into potentially tractable mechanisms of AD pathogenesis, but many questions remain about when, how, and in whom interventions would be indicated.…More
Institute of Neurology, UCL
My concern about this work is that I find it difficult to square with the occurrence of Alzheimer’s disease in all Down's syndrome and in all carriers of some APP and PSEN mutations. The authors in their introduction bring up the idea of “slow virus” diseases. In fact the notion of slow virus diseases was fatally damaged by the identification of prion gene mutations causing these diseases in a hereditary fashion. This meant that, to explain these cases, one had to postulate a universal virus for which mutant prion genes were a universal receptor. In this case, we have to suppose that Down’s cases and the mutation cases either have a totally different disease mechanism or that for some reason, they are uniquely susceptible to these infections.…More
University of Rochester
This is an extremely exciting study that extends our understanding of the role of the human “virome” to neuropathology. The presence of viruses in human brains has long been recognized, but viral detection has been challenging and measurements of serum responses do not accurately reflect the viral load in the brain. In addition, highly neurotropic viruses like those found by the authors often display a stage of latency with periodic and not-well-understood cycles of reactivation and are thus difficult to quantify.
This study tries to tackle these challenges by conducting a large-scale, comprehensive analysis of the association of viral constitutes in Alzheimer disease. The study confirms a previously suggested association of HSV-1 and AD but, unlike previous data, the authors now identify HHV-6A as the most notable virus associated with AD. This is intriguing as HHV-6A has already made a mark as a virus associated with demyelination diseases such as multiple sclerosis (MS). It is tempting to speculate that HHV-6A disrupts fundamentally the same processes in MS and AD, but their consequences are different and might depend on the interplay with the host genome.…More
In addition, HHV-6A is well known for its ability to generate latent infections. What is the difference between the effects of latent and active infection of cells? We showed recently that expression of just a single latency gene product in oligodendrocyte precursor cells is enough to disrupt their migration, a function essential in the repair of myelin damage. Thus, latent infections may also contribute to adverse effects of these common viruses on the progression of AD.
Understanding the potential viral constitutes in the context of neurodegenerative diseases is vital for establishing more “human relevant” animals model. It should not be surprising that animal works often “do not work”—after all, we are ignoring a large amount of biologically active components that co-evolved with humans and that are not simply silent bystanders.
The present study sets the stage for important future work to unlock the biological functions of the human virome.
UC, Irvine
When I first met Frank LaFerla more than 20 years ago at a Keystone meeting, he told me about a herpes simplex virus glycoprotein B fragment with homology to the Alzheimer's Aβ peptide. Frank and I spent some time working on the topic and published a paper in Biochemistry (Cribbs et al., 2000). We are now wondering whether the human herpesvirus 6A (HHV-6A) and human herpesvirus 7 (HHV-7) have homology to Aβ, as well?
References:
Cribbs DH, Azizeh BY, Cotman CW, Laferla FM. Fibril formation and neurotoxicity by a herpes simplex virus glycoprotein B fragment with homology to the Alzheimer's A beta peptide. Biochemistry. 2000 May 23;39(20):5988-94. PubMed.
RIKEN Center for Brain Science
Shingles, induced by activated residual herpes virus, which had previously been silent, in terminal tissues, causes acute symptoms and pain within a few days. If the virus is activated in the CNS, the effect will also be seriously acute. This contradicts the chronic nature of familial and sporadic AD, whose development requires approximately 25 years since initial deposition of Aβ.
In addition, I do not quite trust the pathway analysis. It depends on past publications, many of which have utilized IP-Western experiments and are irreproducible. I thus tend to agree with Charlotte Warren-Gash and John Hardy.
Make a Comment
To make a comment you must login or register.