Immune Cells Clog Capillaries in Mice, Disrupt Memory
Quick Links
One of the characteristic features of Alzheimer’s disease is reduced blood flow in the brain. What brings it down? A paper in the February 11 Nature Neuroscience suggests that white blood cells can clog up the works. Scientists led by Chris Schaffer, Cornell University, New York, discovered that neutrophils—leukocytes that are some of the first responders in the innate immune system—get stuck to vessel walls on their way through capillaries in mouse models of AD. Since cells have to squeeze through, single-file, in these tiny blood vessels, this completely blocks blood flow until the cells work their way free. Even though less than 2 percent of capillaries are affected, their interconnectedness with other vessels means blockages can reduce overall blood flow in the brain by 30 percent. However, antibodies that interfere with the binding of neutrophils to vessel walls restored blood flow and memory quickly, even at advanced stages of disease. The results suggest a possible contributing mechanism to Alzheimer’s.
- Cortical blood flow stalls in AD mouse models.
- Neutrophils wedge themselves in brain capillaries, clogging them up.
- Blocking neutrophil receptors or depleting the cells restores blood flow and memory.
“The small vessels in the brain are often overlooked in the context of AD,” said Susanne van Veluw, Massachusetts General Hospital, Boston. She emphasized how even the smallest changes in capillaries had a significant impact on cerebral blood flow in the mouse brains. “The authors point to a potentially relevant and overlooked mechanism that could contribute to this well-known phenomenon of reduced cerebral blood flow in Alzheimer’s disease.”
Schaffer’s group wanted to study the effects of microvascular injuries on AD pathology. They used transcranial imaging to visualize the vasculature of the mouse brain. They injected fluorescent dye that labeled just the plasma (not red blood cells) of the mice and allowed them to track blood flow after they induced injuries. However, in the process of studying AD mouse controls, they noticed that about 2 percent of capillaries were already blocked, four times more than in wild-type mice. This same obstructed capillary flow has recently been reported in a tau mouse model (Bennett et al., 2018). What was causing this?
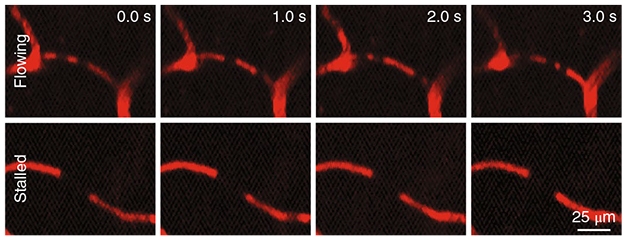
No Flow. In a mouse model of AD, red blood cells moving through capillaries in the brain occlude fluorescence from the plasma (red), appearing as dark spots that move over time (top). In a few percent of vessels, the dark spots hold their position, indicating that blood flow is at a standstill (bottom). [Courtesy of Hernández et al., 2019. Nat Neurosci.]
To examine the phenomenon more closely, first author Jean Cruz Hernández and colleagues first confirmed it happened in several different mouse models of AD. About 1.8 percent of capillaries stalled in APP/PS1 mice as young as 12 weeks old, before plaques had developed. These tiny vessels also clogged in five- to six-month-old 5xFAD mice, and in 10- to 13-month-old TgCRND8 mice. Most plugs lasted less than five minutes, but a third held out for more than 15. The same small subset of capillaries stalled again and again. To identify what caused the obstructions, Hernández tested a series of antibodies to different blood cells and proteins. An antibody to Ly6G, a neutrophil cell-surface marker, labelled almost all the stalled capillaries.
To the authors’ surprise, high doses of the anti-Ly6G label abolished plugs completely within minutes. For both seven- to nine-month-old APP/PS1 animals and five- to six-month-old 5xFAD mice, this boosted blood flow by 30 percent and overall brain perfusion by 20 percent, about two-thirds of the way to wild-type levels. Three hours after treatment, transgenic mice were better able to recognize the location of a new object and remember which arm of a Y-maze they had last explored, demonstrating improvements in spatial and working memory. Though the effects only lasted a couple of days due to neutrophil turnover, repeated doses of anti-Ly6G carried the benefits on for a month. Schaffer has unpublished data suggesting the strategy works for 15- to 16-month-old mice, though not 17- to 20-month-old animals, implying that even at relatively advanced disease stages, improvements in blood flow can improve learning and memory.
What caused the neutrophils to lodge themselves in capillaries? The data aren’t conclusive, but Schaffer strongly suspects that inflammation in the brain and its vasculature driven by Aβ aggregates increases the expression of ICAM1 and VCAM1 on vessel walls (Park et al., 2008). These proteins bind integrin receptors on neutrophils to help them adhere. Basically, the blood vessels become stickier. A previous study reported that anti-Ly6G, through an unknown mechanism, alters the shape of integrins on neutrophils and interferes with adhesion to vessel walls, making the cells themselves less likely to stick (Wang et al., 2012). That may enable them to slide right through the vessel as usual, Schaffer said.
The ties between AD and vascular disease run deep, with countless studies linking poor cardiovascular and cerebrovascular health with greater risk for the disease, and marking it as one of the earliest features of Alzheimer’s (Jun 2014 news; Feb 2012 news; Jul 2016 news). Several reasons have been proposed for the reduced blood flow in the Alzheimer’s brain, including vasoconstriction and loss of vascular density, but scientists don’t fully understand why it occurs (Niwa et al., 2001; Farkas et al., 2001). Could neutrophil stalling be involved? Some drugs that reduce the activation, migration, and adhesion of neutrophils and that are FDA-approved or in clinical trials for autoimmune diseases could help answer this question. However, Schaffer said interfering with neutrophils in Alzheimer’s disease may be unwise because it could compromise the immune system. That said, he is screening some of these drugs to see if they decrease capillary blockages in mice. If one works, a brief clinical trial could help test whether neutrophils reduce cortical blood flow in people. If so, a longer-term strategy might be to target some upstream molecular pathways that lead to the increased adhesion, he speculated.
The study of neutrophils in AD has gained momentum in recent years, noted Gabriela Constantin, University of Verona, Italy. She previously reported that depleting neutrophils improves memory in mouse models of AD (Aug 2015 news). A different study found that fast-declining AD patients have more activated neutrophils than slow decliners or controls (Dong et al., 2018). “This new paper connects neutrophils to capillary stalling, adding a new mechanism by which neutrophils could be involved in disease,” she told Alzforum.
Van Veluw noted that this phenomenon crops up in mice before amyloid pathology appears. “If this is something that happens early in disease and it translates to humans, it could be an interesting target for early intervention,” she said.—Gwyneth Dickey Zakaib
References
News Citations
- Brain Injury Boosts Dementia Risk
- Silent Vascular Disease May Hasten Dementia Progression
- LOAD of Data Place Vascular Malfunction as Earliest Event in Alzheimer’s
- Could Neutrophils Be the Newest Players in Neurodegenerative Disease?
Paper Citations
- Bennett RE, Robbins AB, Hu M, Cao X, Betensky RA, Clark T, Das S, Hyman BT. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer's disease. Proc Natl Acad Sci U S A. 2018 Feb 6;115(6):E1289-E1298. Epub 2018 Jan 22 PubMed.
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1347-52. PubMed.
- Wang JX, Bair AM, King SL, Shnayder R, Huang YF, Shieh CC, Soberman RJ, Fuhlbrigge RC, Nigrovic PA. Ly6G ligation blocks recruitment of neutrophils via a β2-integrin-dependent mechanism. Blood. 2012 Aug 16;120(7):1489-98. Epub 2012 Jun 1 PubMed.
- Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001 Dec;281(6):H2417-24. PubMed.
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001 Aug;64(6):575-611. PubMed.
- Dong Y, Lagarde J, Xicota L, Corne H, Chantran Y, Chaigneau T, Crestani B, Bottlaender M, Potier MC, Aucouturier P, Dorothée G, Sarazin M, Elbim C. Neutrophil hyperactivation correlates with Alzheimer's disease progression. Ann Neurol. 2018 Feb;83(2):387-405. PubMed.
Further Reading
Papers
- Stock AJ, Kasus-Jacobi A, Pereira HA. The role of neutrophil granule proteins in neuroinflammation and Alzheimer's disease. J Neuroinflammation. 2018 Aug 27;15(1):240. PubMed.
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA, Heart-Brain Connection Collaborative Research Group. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017 Aug 22;136(8):719-728. Epub 2017 Jun 6 PubMed.
Primary Papers
- Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, Kang Y, Cortes-Canteli M, Peyrounette M, Doyeux V, Smith A, Zhou J, Otte G, Beverly JD, Davenport E, Davit Y, Lin CP, Strickland S, Iadecola C, Lorthois S, Nishimura N, Schaffer CB. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat Neurosci. 2019 Mar;22(3):413-420. Epub 2019 Feb 11 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Alzheimer center Amsterdam, VU University medical center
With interest we have read the article of Hernández and colleagues investigating the underlying mechanism behind the observation of reduced cerebral blood flow in Alzheimer’s disease.
Cerebral blood flow is an interesting measure by which to investigate the interaction between vascular dysregulation and AD. This study would suggest that there is a link between dysfunction of the vessels and the accumulation of amyloid in the brain, leading to cognitive impairment.
The role of reduced cerebral blood flow in Alzheimer’s disease is still poorly understood. Studies of the association between cerebral blood flow, measured with different modalities (e.g., arterial spin labeling, two-dimensional phase-contrast MRI, or transcranial Doppler), and cognitive function in memory-clinic and population-based studies, showed, in general, modest effect sizes (Leeuwis et al., 2018; Leeuwis et al., 2017; Poels et al., 2008). It would be interesting to investigate at which stage of disease the process of neutrophil plugging in the capillaries begins. Is the interaction of neutrophil in the vessels a terminal event in the pathophysiology of AD or is it an early driver of pathology and cognitive impairment? In addition, it would be interesting to investigate if the same mechanism is found in mouse models with reduced cerebral blood flow due to vascular dysregulation, for example in hypertensive mouse models or mouse models with occlusion of the carotid. Targeting the neutrophil adhesion may be beneficial, as suggested by the cognitive benefit observed in this study.…More
References:
Leeuwis AE, Smith LA, Melbourne A, Hughes AD, Richards M, Prins ND, Sokolska M, Atkinson D, Tillin T, Jäger HR, Chaturvedi N, van der Flier WM, Barkhof F. Cerebral Blood Flow and Cognitive Functioning in a Community-Based, Multi-Ethnic Cohort: The SABRE Study. Front Aging Neurosci. 2018;10:279. Epub 2018 Sep 18 PubMed.
Leeuwis AE, Benedictus MR, Kuijer JP, Binnewijzend MA, Hooghiemstra AM, Verfaillie SC, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease. Alzheimers Dement. 2017 May;13(5):531-540. Epub 2016 Sep 28 PubMed.
Poels MM, Ikram MA, Vernooij MW, Krestin GP, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008 Oct;28(10):1652-5. Epub 2008 Jun 25 PubMed.
University of Southampton School of Medicine
In this elegant study, the authors used in vivo two-photon excited fluorescence (2PEF) microscopy to demonstrate that in APP/PS1, 5xFAD, as well as TgCRND8 mice there is capillary stalling that does not change with advancement of Aβ plaque pathology or in awake vs. anaesthetized mice. The stalling is due to neutrophils and the authors demonstrate that antibodies against Ly6G reduced the stalling within 10 minutes and improved the cerebral blood flow as well as cognition. This is highly significant for future therapeutic targets in Alzheimer’s disease, as targeting the adhesion of leukocytes to the endothelium should be achievable and with potential for significantly improving the clinical picture at mild to moderate stages of the disease, before the walls of blood vessels become compromised (Sweeney et al., 2019). An improvement of cerebral blood flow will not only result in enhanced perfusion of the brain, but also more efficient vasomotion that will likely result in facilitating intramural periarterial drainage (Aldea et al., 2019; Morris et al., 2016). …More
References:
Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Salman RA, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SC, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, González HM, Yuan C, Lockhart SN, Hughes TM, Chen CL, Sachdev P, O'Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MM, Román GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019 Jan;15(1):158-167. PubMed. Correction.
Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front Aging Neurosci. 2019;11:1. Epub 2019 Jan 23 PubMed.
Morris AW, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016 May;131(5):725-36. Epub 2016 Mar 14 PubMed.
Academy of Athens
This is an excellent study providing evidence supporting the significant role of peripheral immune cell populations in AD pathology. Stalled brain capillaries with reduced blood flow seem to play a major role in cognitive function in different AD mouse models. Endothelium inflammation might be a possible cause of neutrophils accumulation in the AD mouse brain.
The authors also suggested that capillary obstruction can happen due to peripheral tissue inflammation. We have observed a similar increase in Ly6C neutrophil population in the brain blood vessels of AD/arthritic mice (5XFAD/huTNF-overexpressing mice) caused by peripheral human TNF. Interestingly, administration of anti-TNF antibody to treat arthritis in the mice, by blocking circulating TNF, decreases Ly6C positive neutrophils in brain capillaries.…More
In our model, peripheral inflammation seems to play a major role in the recruitment of neutrophils in the brain, and modulation of peripheral TNF levels can control this effect. Due to severe arthritis and short life span, cognitive evaluation of 5XFAD/ arthritic mice was not possible (Paouri et al., 2017; Süß, 2017).
We think this is an elegant study with important implications in interpreting the results of different studies on the role of anti-inflammatory drugs in AD. The authors have possibly provided a mechanism explaining the lower risk of AD that is observed in rheumatoid arthritis patients who take anti-TNF drugs, which possibly results in decreased neutrophil recruitment in brain capillaries and improved cognitive function. Of course, a lot of work is still needed to prove if this can be true.
References:
Paouri E, Tzara O, Kartalou GI, Zenelak S, Georgopoulos S. Peripheral Tumor Necrosis Factor-Alpha (TNF-α) Modulates Amyloid Pathology by Regulating Blood-Derived Immune Cells and Glial Response in the Brain of AD/TNF Transgenic Mice. J Neurosci. 2017 May 17;37(20):5155-5171. Epub 2017 Apr 25 PubMed.
Süß P. Remote Control: Impacts of Peripheral Tumor Necrosis Factor-Alpha on Alzheimer Disease-Related Pathology. J Neurosci. 2017 Aug 23;37(34):8045-8047. PubMed.
Make a Comment
To make a comment you must login or register.