In Fruit Flies, MicroRNA Protects Synapses Against Excitotoxicity
Quick Links
With their ability to quickly fine-tune gene expression, microRNAs are a natural fit to regulate synaptic activity in response to the changing needs of the nervous system. Few of these micro-managers are known to work at the presynaptic side, but now researchers have identified a new one, miR-1000. This microRNA has a similar sequence to the mammalian miR-137. It tamps down expression of the glutamate transporter that loads presynaptic vesicles with the neurotransmitter. Glutamate excitotoxicity crippled neurons in flies missing miR-1000, and neurodegeneration ensued, reported senior author Stephen Cohen and colleagues at the Institute of Molecular and Cell Biology in Singapore in the February 2 Nature Neuroscience online.
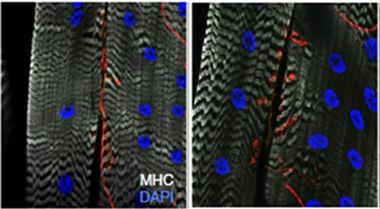
Extra excitation
The neuromuscular junctions of flies lacking miR-1000 (right) contain more and larger synaptic boutons (labeled for VGluT in red) than flies with only one copy of the microRNA gene (left). White indicates the muscle and blue, nuclei. [Image courtesy of Verma et al., Nature Neuroscience.]
MiR-1000 joins several other microRNAs known to participate in synaptic regulation, mostly from studies of rodent hippocampal neurons. Several microRNAs act at the postsynaptic end. For example, three different microRNAs act on synapse strength and glutamate response in rats or mice (Schratt et al., 2006; Saba et al., 2012; Harraz et al., 2012). Less is known about microRNA activity at the presynaptic terminal, though, for example, one microRNA appears to mediate synapse density and glutamate receptor expression in rats (Cohen et al., 2011, reviewed in Kaplan et al., 2013).
Stephen Cohen, who recently moved to the University of Copenhagen, Denmark, was not specifically looking for synaptic microRNAs. He has been screening microRNA knockouts in Drosophila for any kind of neurodegenerative defects, although the researchers have not worked out why (see Oct 2009 Interview). First author Pushpa Verma observed two telltale signs of neurodegeneration in flies lacking miR-1000: They were lackluster climbing the walls of their vials, and they died young. The mutants lived for three weeks after emerging from their pupae as adults, whereas normal flies make it twice as long. To determine if the flies’ neurons were degenerating, Verma examined their brains with an antibody to activated caspase-3, a marker for apoptosis. Sure enough, the brains of 2-day-old adults already contained activated caspase, and 10-day-olds had 10 times as much. Vacuoles also dotted the brains of 10-day-old flies, a sign of age-related neurodegeneration. Verma concluded that the flies underwent early onset, progressive neurodegeneration.
What caused the phenotype? MicroRNAs typically have hundreds of targets, and a computer program predicted that miR-1000 might regulate 374 different genes. Verma focused on just seven known to function in the nervous system. Most microRNAs turn off their targets, and expression of only one, the vesicular glutamate transfer (VGluT) ramped up in miR-1000 knockouts. If excess VGluT was the problem in these mutants, Verma reasoned, then she should be able to fix it by reducing their VGLuT. Sure enough, when she crossed miR-1000 knockouts with flies with diminished VGluT production, she saw a rescue of the phenotype. The double mutants climbed better and lived longer.
One obvious explanation for the neurodegeneration Verma observed was too much glutamate in presynaptic vesicles, leading to excitotoxicity. Verma and Cohen reasoned that if the presynaptic termini were releasing excess glutamate, then reducing glutamate receptor activity on the postsynaptic end should alleviate the problem. First, Verma treated the miR-1000 mutant flies with the receptor blocker memantine. This improved their climbing skills. She also reduced expression of glutamate receptors by knocking out one copy of their genes. Again, this fixed the climbing problems, and extended the flies’ lifespans.
Glutamate signaling should lead to enhanced neural activity. Because in flies glutamate is the neurotransmitter of neuromuscular junctions, and they are relatively easy to dissect, Verma examined them in the miR-1000 mutant larvae. The junctions had more synaptic boutons than normal larvae, and those boutons were bigger (see image above). When she recorded spontaneous activity from the neurons at the junctions, she saw unusually large and frequent action potentials.
Cohen suggested that miR-1000’s normal role is to fine-tune synaptic activity. That does not mean VGluT regulation is miR-1000’s only task. Indeed, the related mammalian miR-137 may be a tumor suppressor that is silenced in some cancers (reviewed in Chen et al., 2013). The particular job of a microRNA often varies by cell type and developmental stage, explained Guoping Feng of the Massachusetts Institute of Technology in Cambridge, who was not involved in the study.
To explore this neurodegeneration mechanism in mammals, Verma depleted miR-137 in mouse primary cortical neuron cultures. The cells made caspase-3, indicating apoptosis. When she depleted miR-137 in the brains of adult mice, they produced more of the mammalian VGluT2.
Does this fly finding explain anything about human neurodegenerative disease? “It doesn’t—yet,” Cohen said. Researchers are still struggling to understand how microRNAs contribute to healthy physiology and disease (see Oct 2009 news). However, there are hints that the neuroprotective mechanism Verma described could, perhaps, be involved in human disorders. MicroRNAs, including miR-137, have been implicated in psychiatric disorders such as schizophrenia, noted Feng (Geaghan and Cairns, 2014; Ripke et al., 2011; Kwon et al., 2013). In addition, one study found unusually low levels of miR-137 in seven brains from people who had Alzheimer’s (see Oct 2011 news).
Glutamate excitoxicity has already been fingered as a possible mechanism in AD as well as Parkinson’s and amyotrophic lateral sclerosis (Ong et al., 2013; Blandini, 2010; Apr 2010 news). Cohen speculated that variants in miR-137, or its target sequence in the glutamate transporter gene, might make a small difference to glutamate loading and cause excitotoxicity that could, over decades, increase risk for neurodegeneration.
That possibility would be worth investigating, commented Fen-Biao Gao of the University of Massachusetts Medical School in Worcester, who did not participate in the study. However, he added that it might be difficult to find the evidence for a miR-137 defect in human postmortem tissues because only certain vulnerable neurons in the brain or spinal cord might have the problem. Looking at the organs as a whole, scientists might miss something, he said.—Amber Dance
References
News Citations
- Amber Dance Interviews Stephen Cohen
- Paris: Macro-roles for MicroRNAs in the Life and Death of Neurons
- Micromanaging Aβ—Small RNAs Control Peptide via Lipids
- Glutamate Gums Up Motor, Dopaminergic Neurons
Paper Citations
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006 Jan 19;439(7074):283-9. PubMed.
- Saba R, Störchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, Schratt GM. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. 2012 Feb;32(3):619-32. Epub 2011 Dec 5 PubMed.
- Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18962-7. PubMed.
- Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11650-5. PubMed.
- Kaplan BB, Kar AN, Gioio AE, Aschrafi A. MicroRNAs in the axon and presynaptic nerve terminal. Front Cell Neurosci. 2013;7:126. Epub 2013 Aug 6 PubMed.
- Chen D, Cabay RJ, Jin Y, Wang A, Lu Y, Shah-Khan M, Zhou X. MicroRNA Deregulations in Head and Neck Squamous Cell Carcinomas. J Oral Maxillofac Res. 2013 Apr 1;4(1):e2. PubMed.
- Geaghan M, Cairns MJ. MicroRNA and Posttranscriptional Dysregulation in Psychiatry. Biol Psychiatry. 2014 Dec 18; PubMed.
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jönsson EG, Bitter I, Pietiläinen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Børglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthøj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jürgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lönnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nöthen MM, O'Dushlaine CT, Olincy A, Olsen L, O'Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Réthelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O'Donovan MC, Daly MJ, Gejman PV, . Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011 Oct;43(10):969-76. PubMed.
- Kwon E, Wang W, Tsai LH. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol Psychiatry. 2013 Jan;18(1):11-2. Epub 2011 Dec 20 PubMed.
- Ong WY, Tanaka K, Dawe GS, Ittner LM, Farooqui AA. Slow excitotoxicity in Alzheimer's disease. J Alzheimers Dis. 2013 Jan 1;35(4):643-68. PubMed.
- Blandini F. An update on the potential role of excitotoxicity in the pathogenesis of Parkinson's disease. Funct Neurol. 2010 Apr-Jun;25(2):65-71. PubMed.
Further Reading
Papers
- Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007 Oct 5;131(1):136-45. PubMed.
- Campbell K, Booth SA. MicroRNA in neurodegenerative drug discovery: the way forward?. Expert Opin Drug Discov. 2015 Jan;10(1):9-16. Epub 2014 Nov 18 PubMed.
- Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009 Apr;32(4):199-206. PubMed.
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013 Jan 5;698(1-3):6-18. PubMed.
News
- Keystone: More Than Mere Nucleotides—miRNAs as Master Regulators, Part 1
- Keystone: More Than Mere Nucleotides—miRNAs as Master Regulators, Part 2
- Do MicroRNAs Cause Mayhem Across Frontotemporal Dementia Spectrum?
- Blocking a MicroRNA Slows Motor Neuron Disease in Mice
- Neurodegeneration and Aging: Could MicroRNA Be the Link?
- Are Upper Motor Neuron Gaffes a Prelude to Disease?
Primary Papers
- Verma P, Augustine GJ, Ammar MR, Tashiro A, Cohen SM. A neuroprotective role for microRNA miR-1000 mediated by limiting glutamate excitotoxicity. Nat Neurosci. 2015 Mar;18(3):379-85. Epub 2015 Feb 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Netherlands Institute for Neuroscience
UK Dementia Research Institute@UCL and VIB@KuLeuven
Glutamate excitotoxicity has been put forward as one of the mechanisms at play in Alzheimer’s disease and other neurodegenerative conditions. In this paper, Verma et al. describe a very interesting microRNA-mediated mechanism for fine-tuning glutamatergic signaling that, when disturbed, can lead to neurodegeneration. They demonstrate how dysregulation of the activity-dependent miR-1000 in Drosophila, and of its homologue miR-137 in mice, controls presynaptic expression of the vesicular glutamate receptor. Increases in these microRNAs lower the glutamatergic tonus in various biological settings, also protecting neurons against excitotoxicity. The authors show that loss of miR-1000 in fruit flies leads to a progressive neurodegenerative phenotype. This paper presents a tremendous amount of elegant work, highly important for our field because it highlights the significance of microRNA “micromanagers” that keep major signaling pathways, relevant to neurodegenerative disorders, in fine-tuned check.
View all comments by Bart De StrooperMichigan State University
This is an interesting study in Drosophila that supports a neuroprotective role for microRNAs in regulating presynaptic glutamate release and protecting against excitotoxicity. The paper highlights the multifaceted targets of microRNAs and reinforces the idea that a number of processes are likely dysregulated leading to neurodegenerative diseases such as AD.
View all comments by Christina ChanVictoria University
Micro-RNAs play a significant role in animal development and in a number of diseases related to neurodegeneration, cancer, and chronic ailments in humans. They are the master regulators of human gene machinery and are small molecules with big impacts, carrying immense therapeutic potential. With the advent of genome-wide studies (next-generation sequencing, small miRNA sequencing, and microarrays), we are beginning to visualize how miRNAs work. While not much is known about the genomic basis of neurodegenerative diseases in humans, genome-wide studies have the potential to provide a holistic view of complex interactions involving miRNAs that may lead to a more profound understanding of these and other diseases that afflict humans. Such holistic approaches are sorely needed for the development of a new generation of genome-based therapies to treat NDs and for possible early correction of functional genetic/functional deficits during developmental stages of an embryo.
In this article, Verma et al. have presented a thought-provoking study in the area of neurodegeneration and developmental biology that may have considerable clinical and scientific implications.
Most exciting is the demonstration that miR-1000 in Drosophila, and its homologue miR-137 in mammals, point to a significant functional analogy among animals. The authors demonstrate that these miRNAs control presynaptic expression of the vesicular glutamate receptor. This has not been shown before and is exciting given that glutamate excitotoxicity is believed to be one of the key mechanisms in the development of Alzheimer’s (AD) and a variety of neurodegenerative diseases (NDs). Although the study is largely relevant to neurodegeneration in Drosophila, it is bound to spark further investigations on NDs in humans, because the findings intersect with genomic and biochemical links to NDs that affect mammals.
Elegant and intelligent experimental design used for generating a panoply of functional mutants of miR-1000 through homologous recombination clearly validated each of the functional phenotypes associated with neurodegeneration, which may be important in the context of NDs in mammals. It was interesting to note that the miR-1000 mutant flies displayed reduced lifespan, with survival declining rapidly by 2 weeks of age, which was rescued by restoring miR-1000 expression using a rescue allele. In climbing performance tests, miR-1000 mutant flies showed early onset of movement disorder, such that by day 10, 80 percent of them had impaired movement. Interestingly, this climbing defect was also corrected and rescued by restoring miR-1000 expression using a rescue allele.
Apoptotic cell death forms the central core of NDs in mammals and the authors probed the role of this neurodegenerative process in Drosophila. They unambiguously demonstrated that cells dying from apoptosis had more caspase-3 positive in miR-1000 mutants as opposed to controls. Again, cell death was rescued by restoring miR-1000 expression using a rescue allele. Further, Drosophila inhibitor of apoptosis protein (DIAP1) reduced the number of caspase-3 positive cells and neuronal death in the mutant brain and improved fly survival, suggesting a link between apoptotic cell death during neurodegenerative disease and reduced life span. Age-related neurodegeneration accompanied by elevated levels of oxidative stress and vacuolization of the Drosophila brain suggests miR-1000 mutants suffered from early onset of neurodegeneration—an analogous scenario to neurodegeneration in mammals. Together, the experiments demonstrate the possible role of miR-1000 in age-related neurodegeneration and its importance in brain developmental stages in Drosophila.
Micro RNAs typically repress their gene targets. Verma et al. used a bioinformatic approach to reveal 374 predicted miR-1000 targets. Although they only analyzed gene targets relevant to the CNS, one of the key genes was the vesicular glutamate transporter, which increased fourfold in RNA isolated from the heads of miR-1000 mutants. This increase was halved in rescued mutants, consistent with partial reduction of miRNA expression. Subsequent deletion of the V-glutamate gene confirmed that overexpression of V-Glut contributed to defects in miR-1000 mutants. Glutamate is the predominant excitatory neurotransmitter in Drosophila CNS and miR-1000 is expressed in presynaptic motor neurons. The authors were able to show that an increase in V-Glut expression was responsible for the increased excitatory synaptic signaling. Memantine, a glutamate receptor antagonist used to treat AD, improved climbing performance in the miR-1000 mutants. Further, reducing NMDA glutamate receptor levels by mutating 1 copy of the Nmdar1 gene improved the survival of miR-1000 mutant flies and suppressed apoptosis. Notably, glutamate receptors are also found in muscles and reducing the muscle-specific glutamate receptors (GluRIIA and IIB) was ineffective, suggesting that these motor problems were a direct effect of glutamate receptors in the brain and that neurodegeneration observed in the miR-1000 mutants could largely be attributed to glutamate excitotoxicity mediated through NMDA, AMPA and metabolic glutamate receptors.
Since it is already known that miR-1000 is not found in mammals, how are these studies by Verma et al. relevant to human disease? Micro RNA-137, a structural homologue of miR-1000, is conserved throughout mammalian species. The homology extends to functional targets. For instance, VGlutT1, T2, and T3 are also predicted to be major targets of miR-137, which is expressed in the dentate gyrus region of the hippocampus in mammals. In a preliminary study, the authors found that VGlutT2 was elevated in the hippocampus on the miR-137-depleted side of the brain.
Finally, the authors show that miR-1000 not only presynaptically regulates VGlut and controls synaptic glutamate release, but also is controlled by light in vivo, presumably reflecting photoreceptor activity in the eye. This, in turn, leads to light-mediated regulation of VGlut receptors levels. What relevance might this have in the context of NDs in mammals? The circadian system, which has a vital role in sleep disorders and wakefulness seen in Alzheimer’s disease and various forms of dementias, is modulated by light. Moreover, it has been shown that light therapy tailored to increase circadian stimulation during the day has benefits for patients with Alzheimer's disease and related dementias living in long-term care facilities. Thus, these studies may have provided a genetic/biochemical lead into this aspect of NDs in mammals, hinting at a potential way to treat it, and should be followed up with more in-depth investigation. Moreover, increased levels of VGlut transporters have been also shown in epilepsy, traumatic brain injury, stroke, and chronic injury. Whether these levels have relevance in the development of vascular dementia remains to be confirmed, as a considerable number of stroke patients experience memory loss and gradual neurodegeneration.
The take-home message from this study is that we need intense investigation at the genome-wide level to better understand the role of miRNAs in mammalian NDs. As a single miRNA can control expression of hundreds to thousands of genes, only large collaborative ventures can determine how the different miRNAs interact with each other and with their cognate targets to modulate function. Such efforts may not only reveal the relevance of miR-137 to human NDs, but may also unveil therapeutically relevant miRNAs that may be used as candidates in treating NDs, leading to tailored therapies for patients in the genomic medicine area.
View all comments by Nitin SaksenaMake a Comment
To make a comment you must login or register.