Does Taming Killer Astrocytes Spare Neurons in Parkinson’s Disease?
Quick Links
Activated microglia contribute to neurodegenerative diseases, and recent work suggests they do that, at least in part, by riling up normally protective astrocytes to attack and kill neurons. Can preventing astrocytes from going bad block the disease? Possibly, says a study in the June 12 Nature Medicine. Seulki Lee, Ted Dawson, and Han Seok Ko, all at Johns Hopkins Medical School in Baltimore, find that in two mouse models of Parkinson’s disease, a diabetes drug prevents the accumulation of toxic synuclein protein in the brain, restores normal movement, and prolongs survival. That was no surprise—similar glucagon-like peptide 1 receptor (GLP-1R) agonists are being tested in clinical trials for Parkinson’s disease. However, when the investigators probed the drug’s mechanism, they found it inhibited microglia activation, and prevented the production of inflammatory cytokines that turn astrocytes into killers.
- Neurodegeneration involves a pathological conversion of astrocytes from protectors to destroyers.
- Diabetes drugs currently in clinical trials for PD and AD block this conversion in two mouse models.
- Astrocytes may provide a novel target.
The new study suggests astrocytes could directly mediate the toxicity of synuclein fibrils in Parkinson’s disease. These angry astrocytes have been implicated in other neurodegenerative diseases, and the results raise the possibility that blocking them could offer a therapeutic approach to multiple conditions.
Co-first authors Seung Pil Yun and Tae-In Kam did not intend to study astrocyte activation. Instead, their goal was to understand how a novel GLP-1R agonist worked in mouse models of Parkinson’s disease. Several GLP-1R agonists are approved for treating Type 2 diabetes, in which they ease insulin resistance and normalize glucose metabolism. In the brain, GLP-1R agonists improve glucose metabolism too, while also stimulating neurogenesis and neuron survival, and quelling inflammation. In a small, Phase 2 study, the GLP-1R agonist exenatide slowed symptomatic progression in people with PD (Aug 2017 news).
In the new study, the researchers first evaluated the activity of NLY01, a long-lived, brain-penetrant GLP-1R agonist developed in Lee’s lab, in two mouse models of PD. In one, mice got injections of preformed α-synuclein filaments into the midbrain. One month later, when synuclein pathology was starting to spread, the researchers began twice-weekly subcutaneous injections of NLY01 or vehicle. After five months, NLY01 had reduced synuclein pathology and dopaminergic neuron loss, and normalized dopamine levels in the mid-brain. The mice showed nearly normal motor function, including running, climbing, grooming, and rearing behaviors. In the second model, in mice expressing the human A53T α-synuclein mutant A53T, NLY01 prolonged the animal’s lifespan by nearly three months, concomitant with a reduction in synuclein pathology at death.
How did the compound work? At the time, Dawson was collaborating with Ben Barres and Shane Liddelow, then at Stanford University in Palo Alto, California, to study astrocyte activation by microglia. They discovered that microglia-derived inflammatory cytokines Il-1α and TNF, along with the complement protein C1q, convert resting astrocytes to a destructive phenotype they called A1 (Jan 2017 news). These inflammatory astrocytes release an unknown factor that participates in dendritic pruning and ultimately causes neuronal death. Liddelow and colleagues had found markers of A1 astrocytes in disease tissue from people with PD, AD, Huntington’s disease, ALS, and MS, and more recently showed they mediated tau toxicity in animal models (Sep 2017 news). Dawson knew that GLP-1R agonists inhibit microglia activation, and wondered if they might save neurons by stopping A1 conversion in response to synuclein. “We did the experiments on a lark, and they turned out to be correct,” he told Alzforum.
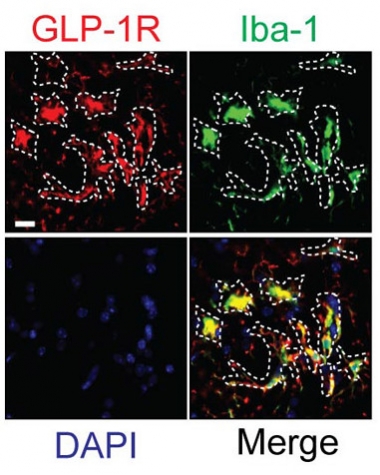
Mainly Microglia.
GLP-1R predominantly co-localizes with the microglial marker Iba-1 in the substantia nigra, supporting a glial locus of action for NLY01 in PD. [From Yun et al., Nature Medicine, 2018.]
To make the case, they first evaluated the effects of NLY01 on isolated microglia, astrocytes, and dopaminergic neurons in culture. NYL01, they found, failed to protect isolated neurons from the toxic effects of α-synuclein fibrils, suggesting that the drug did not act directly on neurons. Microglia in the midbrain expressed more GLP-1R receptors than neurons, and the receptor levels were increased 10-fold in microglia in PD brain tissue, so they tested the effect of NYL101 on those cells. In culture, synuclein triggered activation of microglia, and release of IL-1α, TNFα, and C1q. Conditioned media from synuclein-treated microglia induced A1 markers on astrocytes, which then killed cultured neurons. However, microglia treated with NYL101 failed to release the cytokines, induce A1 astrocytes, or cause neuronal death. Besides NYL01, neutralizing antibodies to Il-1α, TNF, and C1q also eliminated the microglia’s ability to induce A1 astrocytes and neuron death.
The investigators tracked similar events in vivo, where α-synuclein fibril injection or the A53T mutant induced microglia activation, expression of Il-1α, TNF, and C1q RNA, and appearance of complement C3d, a marker for A1 astrocytes (see image). All were inhibited by NLY01. Taken together, the results indicate that microglia-induced astrocyte conversion contributes to neurodegeneration in response to synuclein fibrils, Dawson said.

Can You C3? Injection of mice with synuclein fibrils (top) induces expression of the A1 marker complement 3D (green) in GFAP-positive astrocytes (red, and merged images). Mice who also received NLY01 have fewer C3-positive astrocytes (bottom panels). [From Yun et al., Nature Medicine, 2018.]
“This gives another rodent model of an additional neurodegenerative disease, driven by another protein, but showing the same microglia cascade leading to astrocyte activation and neuronal cell death,” said Liddelow, who is now at New York University. “We had hypothesized this would be a common response, and that seems to be panning out,” he told Alzforum.
Patrik Brundin, Van Andel Research Institute, Grand Rapids, Michigan, called the work a transformative breakthrough, and clinically relevant. But, he said, a few details are puzzling. “It’s a little bit unexpected that the cell death following exposure to synuclein aggregates is so heavily mediated via A1 astrocytes,” he said. “We know from previous work that synuclein is toxic to dopamine neurons in culture. Here, they report that death is heavily dependent on microglia activating astrocytes, and then astrocytes secreting this unknown neurotoxic factor.” Brundin also wondered why NLY01 appeared to decrease the extent of synuclein deposits. “Is that due to protection from the enigmatic neurotoxic factor? It’s unclear why that would reduce spread,” he said.
The work is important for PD researchers because previous preclinical studies with the diabetes drugs came solely from toxin-based PD models, said Thomas Foltynie, University College London. Foltynie ran the initial trials of exenatide for PD, and is planning a Phase 3, pending funding. “Showing the treatment is neuroprotective in two synuclein-based models is important in building confidence that this will help in human PD,” he told Alzforum. He said the study also suggests a potential biomarker for GLP-1R agonist trials, where investigators could monitor microglial activation with PET imaging. At the same time, Foltynie cautions, mice do not always reflect what happens in humans, and the results need to be validated in people first.
NLY01 is on a fast track to human testing. Several of the authors co-founded a company that received funding a month ago and plans to conduct a Phase I tolerability and safety study in healthy volunteers this year, with the goal of a Phase 2 in Parkinson’s disease in 2019.
GLP-1 R agonists are also of interest in AD, where insulin signaling in the brain goes awry and diabetes is a risk factor for disease. One agonist, liraglutide, improved brain glucose metabolism in people with AD (May 2016 news). Will that translate to cognitive benefits? That question is being tested in an ongoing multicenter trial in London. Lee told Alzforum his group is testing NLY01 in animal models of AD.—Pat McCaffrey
References
News Citations
- Diabetes Drug Improves Parkinson’s Motor Symptoms in Small Trial
- Microglia Give Astrocytes License to Kill
- ApoE4 Makes All Things Tau Worse, From Beginning to End
- Diabetes Drug May Rev Up Brain Metabolism in People with Alzheimer’s
External Citations
Further Reading
Primary Papers
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM, Ko HS. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018 Jul;24(7):931-938. Epub 2018 Jun 11 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Milano-Bicocca
Several preclinical studies have tested the potential neuroprotective effects of glucagon-like peptide-1 (GLP-1) analogs in AD, with promising results. In particular, GLP-1 agonists were shown to reduce levels of oligomeric Aβ and plaques, decrease microglial activation, and improve memory behavior. However, the underlying mechanisms of action remain unclear.
It has been recently demonstrated that microglial cells can increase GLP-1 and GLP-1 receptor (GLP-1R) expression in response to inflammatory stimuli, and treatment with the GLP-1R agonist EX-4 was able to reduce brain TNF-α levels in an animal model of AD. Inflammation is increasingly recognized as a key contributor to the pathogenesis of neurodegenerative diseases.
This article describes the protective effects of NLY01, a long-acting GLP-1R agonist, against dopaminergic neuronal loss and behavioral deficits in experimental models of sporadic and familial PD. The relevance of the article lies in the fact that the authors indicate that the mechanism of action is not dependent on neuronal GLP-1R and, for the first time, they suggest a new mechanism involving astrocyte conversion into toxic astrocytes by microglial activation. The paper provides evidence that microglia inhibition mediated by GLP-1 agonist is the primary mechanism of action. These results support that NLY01 and other GLP-1R agonists may have broad protective properties in a variety of neurodegenerative disorders.
Make a Comment
To make a comment you must login or register.