Diabetes Drug Improves Parkinson’s Motor Symptoms in Small Trial
Quick Links
August brought some welcome news for the Parkinson’s community: Results from a Phase 2 clinical trial suggested the diabetes drug exenatide halted the worsening of motor problems in people in moderate stages of the disease. As reported in The Lancet on August 3, motor symptoms slightly improved in those taking the drug for nearly a year, while the placebo group declined. Notably, a portion of the benefits of exenatide, aka Bydureon, persisted for 12 weeks after participants had stopped taking the drug. The results come with caveats, as the trial included only 62 patients, all from a single center. Some commentators were not convinced the results pointed to modification of the disease, as patients had the greatest motor improvements at the beginning of the trial. However, even as the authors and commentators stressed that the findings must be replicated in larger trials, optimism was in the air. Thomas Foltynie of University College London headed the trial.
“I think it is an exciting new era in PD treatment,” commented Ted Dawson of John Hopkins University School of Medicine in Baltimore. “Naturally the trial needs to be replicated in a larger cohort of de novo patients. This should prime the pump for development of this class of drugs for the treatment of PD and related neurodegenerative disease.”
Nigel Greig of the National Institutes of Health in Bethesda, Maryland, a co-author on the paper, called the findings highly promising. “This approved Type 2 diabetes mellitus drug holds potential to impact the course of the disease itself, and not merely the symptoms of PD,” he wrote to Alzforum.
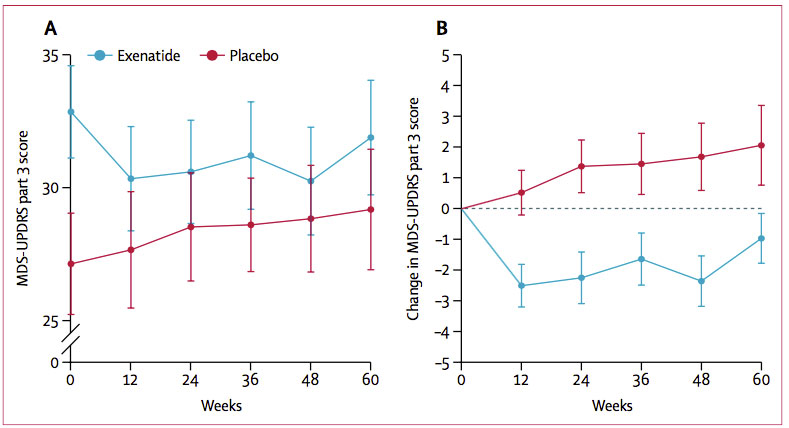
Telling Slopes? Motor scores worsened (increased) in the placebo group (red), and slightly improved in the exenatide group (blue), whose scores were worse at baseline. Left shows absolute scores, right shows change. [Courtesy of Athauda et al., The Lancet, 2017.]
Paul Aisen of the University of Southern California in San Diego did not think the data supported a disease-modifying mechanism. “In general, the results look typical for symptomatic therapy: Rapid improvement followed by a trajectory that parallels the placebo curve,” he wrote to Alzforum. “A larger study with a longer washout period and a predefined delayed start analysis plan would be necessary to address the question of disease modification.”
As a glucagon-like peptide-1 (GLP-1) receptor agonist, exenatide triggers insulin secretion and boosts glucose metabolism. The drug and others in its class are approved for diabetes treatment, but have also assembled a portfolio of neuroprotective benefits in experimental models (for review, see Li et al., 2016). Among them, GLP-1 receptor agonists boost neurogenesis, quell neuroinflammation, and protect dopaminergic neurons from damage inflicted by mitochondrial toxins in animal models of PD (Bertilsson et al., 2008; Cao et al., 2016; Jan 2009 news). This preclinical data motivated researchers to test exenatide in a small, open-label study beginning in 2010. Motor symptoms improved in people with moderate PD who took the drug for a year. This continued two months after exenatide treatment stopped, while patients who took only L-Dopa continued to decline (Jun 2013 news). This proof-of-concept trial lacked a proper placebo group, which the researchers rectified with the current randomized, double-blind, placebo-controlled trial.
First author Dilan Athauda and colleagues conducted the trial at the Leonard Wolfson Experimental Neuroscience Center at University College London. All of the 62 patients had been on dopaminergic treatment, and were starting to experience wearing-off effects of the therapy. Protocol algorithms randomized the volunteers 1:1 to take placebo or 2 mg exenatide, which was self-administered via subcutaneous injection once weekly for 48 weeks. A 12-week washout period, without treatment, followed.
The trial’s primary outcome was a change in scores on Part 3 of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) at 60 weeks, following the 12 week washout. Measurements for this outcome—which were taken every 12 weeks in addition to baseline and 60 weeks—were recorded in the early morning in the “off-medication state,” when patients had not taken their dopaminergic therapy for at least eight hours. Part 3 of the MDS-UPDRS tests motor symptoms, such as tremor, bradykinesia, and gait disturbances. In addition, the researchers measured a number of secondary outcomes, including cognition, quality of life, and mood, when patients were on their normal medications. The researchers also conducted several exploratory measurements, including DaTscan PET to assess dopamine transporter activity, and timed motor tests conducted in both on- and off-medication states. They measured concentrations of the drug in blood and urine every 12 weeks, and checked cerebrospinal fluid for exenatide at 12 and 48 weeks.
Randomization of small trials can produce unbalanced groups by chance, and that was indeed the case with this one. Compared to the 30 participants assigned to the placebo group, the 32 patients randomized to take exenatide were on average four years older, had higher MDS-UPDRS Part 3 scores at baseline, and were taking lower doses of dopaminergic treatment. In this scale, higher scores mean worse motor function.
At 48 weeks, off-medication scores in MDS-UPDRS Part 3 had worsened by 1.7 points in the placebo group, while participants taking exenatide had improved by 2.3 points compared to baseline. At 60 weeks, the placebo group had declined by 2.1 points, while volunteers taking exenatide maintained an improvement of 1 point better than baseline. Therefore, while the placebo group steadily declined throughout the course of the trial, the exenatide group got better, and maintained a portion of that benefit even after 12 weeks without the drug. While the exenatide group outperformed their baseline scores at 60 weeks, their scores did slip after stopping treatment.
Notably, in keeping with the worse baseline motor performance in the exenatide group, the placebo group outperformed the treatment group throughout the trial. Athauda emphasized to Alzforum that a change in scores, rather than a difference in absolute scores between groups, was the predefined primary outcome measure. Other commentators agreed that the worse average scores in the treatment group did not detract from the study’s main finding: that motor scores actually improved in people taking exenatide.
“Changing the slope of that curve is the Holy Grail of PD research,” commented Patrik Brundin of the Van Andel Institute in Grand Rapids, Michigan. He added that because both the exenatide and placebo groups started the trial 6.4 years after their PD diagnosis, the poorer baseline motor scores of the exenatide group suggest they had a more rapidly progressing disease. This makes their improvement all the more impressive and convincing, he said.
Though participants taking exenatide clocked a net improvement in motor scores between baseline and 60 weeks, most of the gain occurred during the first 12 weeks, and waned as the trial went on. Brundin speculated that acute pharmacological effects, such as enhanced dopamine production, could be responsible for this initial burst of improvement. He added that other effects of the drug, such as neuronal repair, neuroprotection, and quenching damaging neuroinflammation, may exert benefits more gradually.
According to Christian Hölscher of Lancaster University in England, the finding that exenatide’s benefits outlasted the 12-week washout period suggests the trial achieved the ultimate of outcomes: disease modification. “This is proof that exenatide makes a long-term impact, as opposed to just patching up symptoms,” he told Alzforum. “We’ve finally struck gold.”
Not everyone was convinced. Suzanne Craft of Wake Forest School of Medicine in Winston-Salem, North Carolina, took a more cautious tone, pointing out that motor scores started to deteriorate after treatment stopped. “A longer follow-up period would be helpful in determining whether there is a rebound effect, or whether there is truly some persistent benefit to exenatide treatment that might support a disease modifying mechanism,” she wrote to Alzforum.
David Standaert of the University of Alabama at Birmingham felt there could be other explanations as well. “While the improvement in ‘off-medication’ state could be a disease-modifying effect, it could also be a long-lasting symptomatic effect, or an effect on the pharmacodynamics of the other medications.”
The authors themselves were also conservative in their interpretation of the results. “It might be tempting to view persistent benefits detectable after the washout period as evidence of disease modification,” they wrote in the paper’s discussion. However, they noted that 12 weeks may have been insufficient time to eliminate unexpected long-lasting symptomatic effects. For example, symptomatic relief may promote participants to take up or maintain healthy behaviors such as exercise, which could have long-term benefits.
Despite improvements on the MDS-UPDRS Part 3, no significant changes on secondary outcome measures were noted, including quality of life. This is not entirely surprising, Brundin said, given the short duration of the trial, small motor improvements, and relatively moderate symptoms the participants started out with. He and Hölscher speculated that more noticeable benefits could emerge in longer trials.
Exenatide also did not boost motor performance in participants while they were under the influence of dopaminergic treatment. The failure of this exploratory measure indicates that exenatide only affects symptoms when participants are in an “artificial state,” namely when they are not taking their usual medications, Standaert pointed out.
As another exploratory outcome, the researchers measured dopamine transporter activity via DaTscan at baseline and 60 weeks. They found that while activity in dopaminergic regions declined in both groups, it did so significantly less in the exenatide group. In a separate commentary co-authored with Brundin and Richard Wyse of Cure Parkinson’s Trust in London, the authors cautioned against over-interpretation of these imaging results, pointing out that they were not adjusted for multiple comparisons (Athauda et al., 2017).
Exenatide seemed well-tolerated. Adverse events—including gastrointestinal symptoms, weight loss, nausea, and loss of appetite—occurred with similar frequency in the treatment and placebo groups. People in the exenatide group lost more weight on average than those in the placebo group, but the researchers observed no significant correlation between the degree of weight loss and primary outcome. Of the eight serious adverse events that occurred throughout the trial, six were in the exenatide group, though the authors stated that none were considered related to treatment. One person had to discontinue exenatide due to elevation in the pancreatic enzyme amylase at 12 weeks. Hyperamylasemia has been documented in diabetics taking the drug, but caused no symptoms in the patient in this trial.
Regardless of their interpretation of the results, all authors and commentators Alzforum consulted called for a longer, multicenter Phase 3 trial of the drug in PD patients. The source of funding for such an expensive trial is up uncertain, especially given that AstraZeneca’s patent on exenatide recently expired. The Michael J. Fox Foundation sponsored the present trial. Because this drug and others in its class are already approved for diabetes treatment, it is possible that some doctors could prescribe them off-label to people with PD. However, Brundin and other commentators emphasized that most physicians would be appropriately unwilling to do so without further proof of the drug’s effects on the disease.
Standaert, a clinician, agreed, pointing to the need for larger trials. “Outside of a clinical trial, I would not recommend treatment with exenatide to any of my patients with PD at this time. The benefits are uncertain at best, and there are clearly risks of therapy.”—Jessica Shugart
References
News Citations
- Type 2 Diabetes and Neurodegeneration—The Plot Caramelizes
- Single-Blind Trial: Diabetes Drug Helps Parkinson’s, Maybe
Paper Citations
- Li Y, Li L, Hölscher C. Incretin-based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev Neurosci. 2016 Oct 1;27(7):689-711. PubMed.
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H, Wikström L. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008 Feb 1;86(2):326-38. PubMed.
- Cao L, Li D, Feng P, Li L, Xue GF, Li G, Hölscher C. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson's disease by reducing chronic inflammation in the brain. Neuroreport. 2016 Apr 13;27(6):384-91. PubMed.
- Athauda D, Wyse R, Brundin P, Foltynie T. Is Exenatide a Treatment for Parkinson's Disease?. J Parkinsons Dis. 2017;7(3):451-458. PubMed.
Other Citations
External Citations
Further Reading
Primary Papers
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 3; PubMed.
- Bassil F, Canron MH, Vital A, Bezard E, Li Y, Greig NH, Gulyani S, Kapogiannis D, Fernagut PO, Meissner WG. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017 Mar 14; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging, National Institutes of Health
This Dilan Athauda/Tom Foltynie Lancet article evaluating the GLP-1 agonist exenatide in Parkinson’s disease (PD) patients in a randomized, double-blind, placebo-controlled trial provides a highly promising finding, in that this approved Type 2 diabetes mellitus drug holds potential to impact the course of the disease itself, and not merely the symptoms of PD. Currently available treatment strategies can mitigate many of the symptoms of PD over several years, but—similar to Alzheimer’s disease (AD)—the disease continues to progress and worsen. Hence, for both disorders, finding and developing well-tolerated drugs that reduce disease progression is imperative.
The results from this exenatide PD clinical trial, together with those of Tom Foltynie and colleagues’ prior study (Aviles-Olmos et al., 2013) and a huge number of preclinical studies that include our own (reviewed in Kim et al., 2017) fully support continued clinical evaluation of exenatide in PD over a longer duration and in well-designed, multicenter clinical trials with the potential to recruit more participants. With our increasing knowledge of the synergistic mechanisms via which exenatide and related incretin mimetics appear to inhibit pathways that drive disease progression in cellular and animal models of PD, it would clearly be valuable to know whether such pathways are affected in PD patients administered exenatide. This can potentially be evaluated in well-designed biomarker studies, and provide insight into which PD patients might better respond to exenatide.
Finally, many of the pathways that exenatide and incretin mimetics positively impact are relevant across neurodegenerative disorders—both acute, like traumatic brain injury and stroke (Tweedie et al., 2013; Greig et al., 2014), and chronic, like AD and multiple system atrophy (Hölscher 2014; Basil et al., Brain 2017, in press), and hopefully the recent positive results from this exenatide PD trial will encourage the consideration of clinical trials in these disorders that, like PD, lack effective treatments.
References:
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013 Jun 3;123(6):2730-6. PubMed.
Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A New Treatment Strategy for Parkinson's Disease Through the Gut-Brain Axis: The Glucagon-like Peptide-1 Receptor Pathway. Cell Transplant. 2017 Apr 26; PubMed.
Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013 Jan;239:170-82. Epub 2012 Oct 8 PubMed.
Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, Sambamurti K, Becker RE, Pick CG. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014 Feb;10(1 Suppl):S62-75. PubMed.
Hölscher C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer's disease. Alzheimers Dement. 2014 Feb;10(1 Suppl):S47-54. PubMed.
University of Alabama at Birmingham
This is an interesting study that has prompted a lot of discussion among both scientists and patients. It is a relatively small-scale trial, and enrolled people with moderate to advanced PD who were already on levodopa and a number of other medications. The only effect they saw was a change in the UPDRS 3 measured after being off their medications overnight. I would not call this effect “significant motor improvement,” as it was seen only under the artificial circumstance of withdrawing other medications. In terms of day-to-day “on” state motor symptoms, exenatide did not demonstrate any significant improvement over conventional treatment.
The deeper question here is whether exenatide has any long-term disease-modifying effects. Preclinical studies have suggested this is possible, but the current trial doesn’t resolve this question. While the improvement in “off” state could be a disease-modifying effect, it could also be a long-lasting symptomatic effect, or an effect on the pharmacodynamics of the other medications. There were also some significant side effects, including weight loss. These are a concern, but at least at present don’t seem to be a barrier to further study of exenatide.
On the whole, I think this study accomplished what is possible in a trial of relatively small scale—it established reasonable safety and tolerability, and provide the parameters needed for a larger-scale trial to address the question of long-term disease modifying effects.
And to answer the question I always get from my patients—outside of a clinical trial, I would not recommend treatment with exenatide to any of my patients with PD at this time. The benefits are uncertain at best, and there are clearly risks of therapy.
Henan University of Chinese Medicine
This is a very promising result that shows that the concept of using growth factors such as GLP-1 that can cross the BBB does improve progressive neurodegenerative conditions such as Parkinson's disease. We should keep in mind that all efforts to affect disease progression in AD or PD have failed, and here we see a clear result in improving the condition. When seen in the context of other positive outcomes of clinical trials, such as the nasal insulin treatment of Alzheimer's patients by Suzanne Craft and colleagues and the positive outcome ot the liragutide trial by Gejl et al., 2016, showing a protection of progressive neurodegeneration in18-FDG-PET imaging, it should be clear by now that this approach does genuinely improve the condition. By how much we do not know, but other clinical trials that test liraglutide or lixisenatide (both GLP-1 receptor agonists) are on the way. The main takeaway message from this trial is that we have found a target that does show clear improvements, and that is worth exploring further. We finally have a strategy that shows clinical improvements, which we were looking for in the last 60 years without success!
References:
Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, Møller N, Brock B, Rungby J. In Alzheimer's Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci. 2016;8:108. Epub 2016 May 24 PubMed.
Make a Comment
To make a comment you must login or register.