Death by Goo: TDP-43 Gels Paralyze Proteasomes in Neurons
Quick Links
Like a woolly mammoth in a tar pit, proteasomes may be doomed if they get stuck in gobs of the DNA-binding protein TARDP, better known as TDP-43. That’s according to new research from the labs of Dieter Edbauer at the German Center for Neurodegenerative Diseases, Munich, and Rubén Fernández-Busnadiego at the University of Göttingen, Germany. The scientists used cryo-electron tomography, proteomics, and functional assays to show that fragments of TDP-43 form gel-like amorphous blobs in the cytosol, and that these are enriched with proteasomes stuck in mid-catalysis. The authors believe this may be a common pathological mechanism in amyotrophic lateral sclerosis and frontotemporal dementia, which can be caused by mutations in TDP-43.
- C-terminal fragments of TDP-43 form gel-like inclusions.
- These ensnare stalled proteasomes.
- This might be a common pathological mechanism in ALS/FTD.
Insoluble inclusions of TDP-43 are found in the brains of people with not only ALS/FTD but other neurodegenerative diseases, as well, including Alzheimer’s and LATE, aka limbic predominant age-related TDP-43 encephalopathy (May 2019 news). Recently, scientists used cryo-electron microscopy to determine the structure of TDP-43 fibrils, but the protein also forms liquid droplets through a process called liquid-liquid phase separation (Dec 2021 news; Sep 2016 news). Though rare, LLPS explains amorphous lumps formed by proteins that have limited secondary structure, including TDP-43, FUS, tau, and other proteins linked to neurodegeneration. Comprising an extensive low-complexity domain, the C-terminal end of TDP-43 drives LLPS, and it is precisely the C-terminus of this predominantly nuclear protein that accumulates in the cytosol in ALS/FTD.
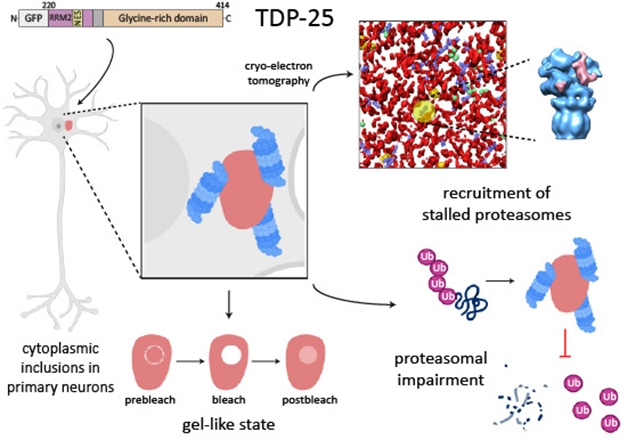
Pity the Proteasome. In primary neurons, 25kDa C-terminal stubs of TDP-43 form gel-like blobs (red), which contain ring-like structures (yellow) formed by proteasomes (blue). These are bound to an unknown structure (pink) that might be a substrate or an inhibitor. [Image courtesy Riemenschneider et al., EMBO Reports.]
To study these fragments and their role in disease, co-first authors Henrick Riemenschneider from the DZNE and Qiang Guo from the Max Planck Institute of Biochemistry, Martinsried, also in Germany, turned to cryo-electron tomography, a type of cryo-EM that can render high-resolution images of complex structures in situ. It can uncover structures of membrane proteins in cellulo, and Guo previously used it to discover that ribbons of the poly dipeptide polyGA ensnare proteasomes (Feb 2018 news).
Looking in primary rat cortical neurons, the scientists found that TDP-25, a 25kDa C-terminal fragment of TDP-43, forms blobs that do the same. Both wild-type TDP-25 and fragments carrying any of eight known TDP-43 mutations linked to ALS blobbed up in this manner. Cryo-ET also revealed ring structures in these blobs that were proteasomes. Based on the structure, the researchers concluded that all the trapped proteasomes were in the midst of degrading substrates. This is unusual. In a normal neuron, only about 20 percent of proteasomes would be in a substrate processing state, with the majority cycling through a “ground” state. The authors believe that the proteasomes in the TDP-25 gels have, in fact, stalled.
To test this, they expressed TDP-43 variants in HEK293 cells that express a proteasome reporter, namely a ubiquitin mutant/green fluorescent protein chimera. Proteasomes readily degrade UbG76V-GFP, as they did in cells expressing full-length TDP-43; alas, in cells expressing even a small fraction of wild-type TDP-25, the substrate began to accumulate. ALS mutations in TDP-25 impaired proteasome activity even further.
TARDP Pit? Cryo-ET rendering neuronal cytosol shows proteasomes (violet) and some chaperonins (green) mired in a pit of TDP-25 (red). Ribosomes (yellow) are banished to the periphery. [Movie courtesy Riemenschneider et al., EMBO Reports.]
Interactome analysis supports the idea that cytosolic TDP-43 fragments interact with the proteasome. The scientists found a huge difference between partners of full-length TDP-43 and TDP-25. The latter ignored 72 normal partners, mostly those involved in RNA splicing, but bound 400 new ones. These included proteasome subunits and other proteins linked to the ubiquitin-proteasome system (UPS), such as ubiqulin 2 and E3 ubiquitin ligases. The authors speculate that these might contribute to an unknown density in the proteasome spotted by cryo-ET (see move above).
All told, the authors concluded that “TDP-43 C-terminal fragments adopt a gel-like conformation and impair proteostasis by sequestering stalled proteasomes in situ.” The similarity with their previous findings on polyGA suggests this might be a common problem in ALS/FTD. ALS-causing mutations have also been found in other genes that are linked to the UPS, including ubiquilin 2, VCP, OPTN, C9orf72, and TBK1.—Tom Fagan
References
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Riemenschneider H, Guo Q, Bader J, Frottin F, Farny D, Kleinberger G, Haass C, Mann M, Hartl FU, Baumeister W, Hipp MS, Meissner F, Fernández-Busnadiego R, Edbauer D. Gel-like inclusions of C-terminal fragments of TDP-43 sequester stalled proteasomes in neurons. EMBO Rep. 2022 Jun 7;23(6):e53890. Epub 2022 Apr 19 PubMed. Correction.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.